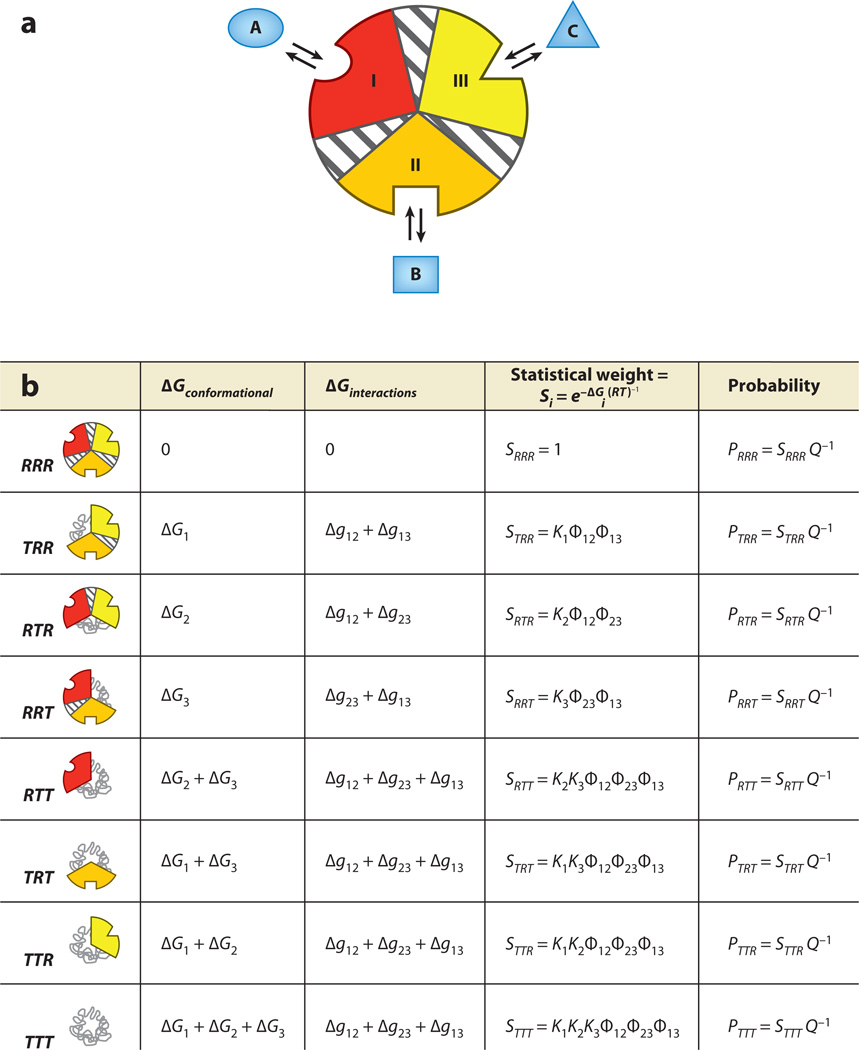
Figure 6.
The ensemble allosteric model applied to a three-domain allosteric protein. (a) Three domains (possibly subdomains) within a single hypothetical molecule. Each domain is specific to a separate ligand, and each subdomain contains an energetic interaction between the two remaining subdomains. As for the two-domain model, each conformational state may be thought of as tense (low affinity) or relaxed (high affinity), which may possibly be related to the folded state of the domain. Thus, microstate 1, where domain I is low affinity and domains II and III are high affinity, is abbreviated TRR. (b) Free energies and Boltzmann-weighted populations of each microstate in the ensemble. Note that this three-domain model is a simple mathematical extension of that developed above.