Abstract
Importance
The default mode network (DMN) is a collection of brain regions that reliably deactivate during goal-directed behaviors and is more active during a baseline, or so-called resting, condition. Coherence of neural activity, or functional connectivity, within the brain’s DMN is increased in major depressive disorder relative to healthy control (HC) subjects; however, whether similar abnormalities are present in persons with dysthymic disorder (DD) is unknown. Moreover, the effect of antidepressant medications on DMN connectivity in patients with DD is also unknown.
Objective
To use resting-state functional-connectivity magnetic resonance imaging (MRI) to study (1) the functional connectivity of the DMN in subjects with DD vs HC participants and (2) the effects of antidepressant therapy on DMN connectivity.
Design
After collecting baseline MRI scans from subjects with DD and HC participants, we enrolled the participants with DD into a 10-week prospective, double-blind, placebo-controlled trial of duloxetine and collected MRI scans again at the conclusion of the study. Enrollment occurred between 2007 and 2011.
Setting
University research institute.
Participants
Volunteer sample of 41 subjects with DD and 25 HC participants aged 18 to 53 years. Control subjects were group matched to patients with DD by age and sex.
Main Outcome Measures
We used resting-state functional-connectivity MRI to measure the functional connectivity of the brain’s DMN in persons with DD compared with HC subjects, and we examined the effects of treatment with duloxetine vs placebo on DMN connectivity.
Results
Of the 41 subjects with DD, 32 completed the clinical trial and MRI scans, along with the 25 HC participants. At baseline, we found that the coherence of neural activity within the brain’s DMN was greater in persons with DD compared with HC subjects. Following a 10-week clinical trial, we found that treatment with duloxetine, but not placebo, normalized DMN connectivity.
Conclusions and Relevance
The baseline imaging findings are consistent with those found in patients with major depressive disorder and suggest that increased connectivity within the DMN may be important in the pathophysiology of both acute and chronic manifestations of depressive illness. The normalization of DMN connectivity following antidepressant treatment suggests an important causal pathway through which antidepressants may reduce depression.
Dysthymic disorder (DD) is debilitating and common, affecting 2.5% to 5% of the US population.1 Patients often struggle with significant functional impairments including unemployment, high health care use, and the use of entitlements such as Supplemental Security Income/Social Security Disability.2 As with major depression, effective treatments are available for DD, yet up to half of depressed patients either do not respond or drop out of treatment prematurely.3 More effective treatments with fewer adverse effects are sorely needed. However, the development of better treatments is impeded by at least 2 principal knowledge gaps: (1) limited understanding of the pathophysiology of DD and (2) incomplete knowledge of the causal mechanisms by which existing interventions are effective.
To our knowledge, only 3 prior studies have used magnetic resonance imaging (MRI) to study patients with a primary diagnosis of dysthymia,4–6 contrasting with the numerous neuroimaging studies of major depressive disorder (MDD).7–11 Moreover, these studies were limited by small and/or heterogeneous samples. Regarding treatment, firstline agents for depressive illnesses include selective serotonin reuptake inhibitors and serotonin and norepinephrine reuptake inhibitors. The neurochemical effect of these agents is well described—they constrain the uptake of monoamines at the synaptic cleft—yet, the mechanism by which this neurochemical effect leads to salutary, clinical effects is poorly understood.
We aimed to investigate (1) the pathophysiology of DD and (2) causal mechanisms by which antidepressants are effective in treating DD. We did this by collecting resting-state (RS) functional-connectivity (FC) MRI scans in a population of adults with DD before and after treatment in a double-blind, placebo-controlled trial of duloxetine, a serotonin and norepinephrine reuptake inhibitor. We focused on the brain’s default mode network (DMN), a collection of brain regions that reliably deactivate during goal-directed behaviors.12 Neural activity in one brain region within the DMN strongly correlates over time with activity in other brain regions within the DMN. We used RS-FC MRI to examine these interregional functional connections13 in the DMN in persons with DD for 3 principal reasons. First, the DMN is thought to underlie the mental process of introspection—the mind turning inward as it moves away from externally focused thoughts. Because excessive introspection in the form of rumination is a well-described feature of depression in general and of dysthymia in particular,14,15 we hypothesized that the functional architecture of the DMN might be altered in dysthymia.16 Second, because prior studies have described abnormal function and connectivity of the DMN in adults with MDD,7,17 we aimed to determine whether this abnormality generalizes to dysthymia—a chronic form of depression—or is specific to major depressive episodes—an acute form of depression. Third, serotonin receptor density modulates the functioning of the DMN18 and tryptophan depletion alters DMN connectivity,19 suggesting that the DMN may represent an important site of action for serotonergic antidepressants. Our principal hypotheses were that DD relative to healthy control (HC) participants would demonstrate greater DMN connectivity, and that this abnormal DMN connectivity would normalize with duloxetine treatment.
METHODS
The institutional review board of the New York State Psychiatric Institute approved the study procedures. Participants provided written, informed consent.
PARTICIPANTS
Our cohort comprised 41 adults with DD and 25 HC subjects between 18 and 53 years of age. All participants were free of significant medical problems. Patients fulfilled DSM-IV-TR criteria for DD and were excluded if they were currently experiencing a major depressive episode. Patients with comorbid Axis I disorders, other than anxiety disorders, were excluded from the study. Patients with prior medication treatment, a prior major depressive episode, and/or comorbid anxiety disorders were included in the study; these data were recorded (Table 1) and included as covariates in subsequent analyses. Of the 41 patients with DD in the study, 19 (46%) had a history of treatment with psychotropic medications, but none of the participants were taking psychotropics within a week of their MRI scan (4 weeks for fluoxetine). Control subjects were assessed for psychiatric disorders using the Structured Clinical Interview for DSM-IV20; had no significant, active Axis I disorder; were medication free; and were group matched to patients with DD by age and sex (Table 1).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| No. | |||||
|---|---|---|---|---|---|
| Dysthymic Participants (n = 41) |
Healthy Control Subjects (n = 25) |
Test Statistic | P Value | ||
| Age, mean (SD), y | 37.8 (9.0) | 33.0 (11.9) | t = 1.9 | .07 | |
| Sex | |||||
| Male | 24 | 17 | χ2 = 0.6 | .44 | |
| Female | 17 | 8 | |||
| Handedness | |||||
| Right | 38 | 24 | χ2 = 0.3 | .58 | |
| Left | 3 | 1 | |||
| Highest level of education | |||||
| College or greater | 25 | 18 | Mann-Whitney U = 375.0 | .06 | |
| High school | 14 | 4 | |||
| Below high school | 0 | 2 | |||
| Not specified | 2 | 1 | |||
| Race/ethnicity | |||||
| White | 30 | 19 | χ2 = 5.5 | .20 | |
| African American | 9 | 3 | |||
| Hispanic | 1 | 1 | |||
| Asian | 1 | 0 | |||
| Not specified | 0 | 2 | |||
| Anxiety disorder | |||||
| Current | 16 | 0 | |||
| Past | 12 | 0 | |||
| Prior episode of MDD | 19 | 0 | |||
| Prior substance abuse | 11 | 0 | |||
Abbreviation: MDD, major depressive disorder.
Diagnoses were made using clinical interviews of a board certified psychiatrist and confirmed with the Structured Clinical Interview for DSM-IV.20 Control subjects completed a single MRI scan; participants with DD completed baseline (ie, pretreatment) and follow-up (ie, posttreatment) MRI scans. Prior to scanning, a trained rater assessed depressive symptoms using the 24-item Hamilton Depression Rating Scale (HAM-D).21
MRI PULSE SEQUENCES
Images were acquired on a GE Signa 3-T whole-body scanner. T1-weighted sagittal localizing images were acquired, followed by a 3-dimensional spoiled gradient recall image for coregistration with axial echoplanar images. Axial echoplanar images (repetition time, 2200 milliseconds; echo time, 30 milliseconds; 90° flip angle; receiver bandwidth, 62.5 kHz; single excitation per image; slice thickness, 3.5 mm; slices per volume, 34; 24 × 24 cm field of view; 64 × 64 matrix) were obtained to provide an effective resolution of 3.75 × 3.75 × 3.5 mm and whole-brain coverage. For RS acquisition, participants were instructed to remain still with their eyes closed and to let their minds wander freely. Two 5-minute RS scans were obtained for each participant. The same imaging procedures were used for baseline and follow-up scans.
CLINICAL TRIAL
After completion of the baseline MRI scan, participants with DD began a 10-week double-blind, placebo-controlled study of duloxetine therapy at the Depression Evaluation Service of the New York State Psychiatric Institute. A full description of the clinical trial is provided elsewhere22 and the results are summarized in eAppendix 1 (http://www.jamapsych.com). Briefly, subjects were randomly assigned to double-blind treatment with duloxetine or placebo. Dosing began at 30 mg daily and could be increased to a maximum of 120 mg daily in the absence of sufficient response (defined as a Clinical Global Impression–Improvement score <2). Of the 41 participants with DD who completed the baseline MRI scan, 32 completed the randomized clinical trial and follow-up scan.
IMAGE PROCESSING
Personnel were blinded to the treatment arms during image processing. Standard image preprocessing methods were used, with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and the conn toolbox (http://www.nitrc.org/projects/conn) for FC analysis. The functional images were motion corrected, coregistered with a high-resolution anatomic scan, normalized into the Montreal Neurological Institute space, resampled at 2 mm3, and smoothed with a Gaussian kernel of 6 mm3 full-width at half maximum.23 Connectivity preprocessing procedures followed the component-based noise-correction method described elsewhere24 to minimize nonneural influences on functional MRI (fMRI) signal (eAppendix 2). To minimize the likelihood that imaging findings were the product of unspecified confounds or artifacts, we conducted a negative control analysis by analyzing connectivity maps derived from a seed region within the primary motor cortex (eAppendix 3 and eFigure 1).
Following preprocessing, the RS data were correlated voxel by voxel for each participant across the complete resting time series. Fisher z transformation was applied. We generated connectivity maps for each subject, with the seed region defined by the mean fMRI signal from voxels within the posterior cingulate cortex (PCC). We selected the PCC as the seed region because of its key role in the DMN and its reliable identification of the DMN in connectivity studies.25–27 A spherical mask centered at the PCC (and extending into the precuneus) is provided by the conn toolbox and is derived from the PCC coordinates described by Fox et al.28
NETWORK ANALYSIS
We implemented social network analysis (graph theory29) to provide an additional test of our hypothesis that compared with HC subjects, participants with DD have greater FC within the DMN. We did this by extracting the blood oxygenation level–dependent time series data for 12 a priori regions of interest (ROI) within the DMN. The ROIs were first identified by Fox et al,28 who delineated 13 DMN ROIs including 1 ROI within the cerebellum. Owing to inconsistent coverage of the cerebellum, we did not include the cerebellar ROI in our analysis. The same approach has been used in prior connectivity studies of the DMN.30,31 The DMN ROIs and their stereotactic coordinates are delineated in eTable 1. The time series data for each ROI were correlated region by region for each participant, producing a single 12 × 12 correlation matrix per subject. We calculated the network density (defined as the mean of all possible network connections)32 using UCINET,33 software designed for social network analysis. This procedure reduces each subject’s 12 × 12 correlation matrix into a single variable that indexes the global connection density for the DMN. Two principal approaches allow calculation of network density34—valued and nonvalued graphs (a full description of these 2 approaches is provided in eAppendix 4). Both approaches have merit and potential weaknesses, thus we performed both. Results from the valued and nonvalued graphical approaches were comparable. We present the valued graphical analyses here and the nonvalued graphical analyses in the online-only material (eTable2).
HYPOTHESIS TESTING
We conducted separate hypothesis testing for the baseline MRI scans (comparing subjects with DD vs HC participants) and the follow-up scans (comparing changes in the MRI scans of duloxetine-treated vs placebo-treated participants with DD). The analyses could not be reduced into a single model because comparing subjects with DD vs HC participants required a between-subject analysis, whereas a repeated-measure analysis was necessary to compare baseline vs follow-up MRI scans from the participants with DD. Additionally, because our hypotheses were specific to the DMN, our group-level analyses only reflect regions that were positively correlated with the PCC; we did not include regions inversely correlated with the PCC (ie, task-positive networks).
BASELINE MRI SCANS
To test the hypothesis that participants with DD have greater DMN connectivity than HC subjects, we entered each participant’s seed-based connectivity map into a second-level, random-effects factorial model using SPM8. We treated group as the single factor with 2 levels: subjects with DD and HC participants. We performed additional analyses that included prior medication exposure, history of major depressive episodes, substance abuse, and the presence of a comorbid anxiety disorder as additional factors in the second-level, factorial models.
The network density analyses reduced each subject’s DMN connectivity into a single variable that indexed the global connection density for the DMN. We compared the connection densities across the 2 groups (ie, DD vs HC) using a 2-sample t test. Additional analyses of covariance included prior medication exposure, history of major depressive episodes, substance abuse, and the presence of a comorbid anxiety disorder as covariates.
FOLLOW-UP MRI SCANS
We tested our hypothesis that DMN connectivity would be normalized in the duloxetine-treated, but not placebo-treated, patients with DD. For the voxelwise analysis, we entered each participant’s seed-based connectivity map into a 2 × 2 repeated-measure factorial model using SPM8. We treated time as a repeated measure with 2 levels: baseline scan and follow-up scan; we used treatment as a between-group factor with 2 levels: duloxetine and placebo. We isolated an interaction term (time × treatment) to determine differential effects of treatment on connectivity. We then conducted post hoc t tests to determine the nature of the interaction. Because the duloxetine arm included fewer men than the placebo arm, we included sex as a covariate (eTable 3). The 2 treatments were comparable on other demographic and clinical characteristics (eTable 3). However, given the large number of statistical tests in a voxelwise whole-brain analysis, we reasoned that baseline connectivity differences between the duloxetine and placebo arms could be present owing to chance alone. Baseline differences in connectivity could produce spurious interpretations of treatment × time interactions. To preclude this possibility, we compared the baseline scans in the duloxetine vs placebo arms by conducting an f test on the PCC connectivity maps with an uncorrected α of less than 0.05. The results are presented in eAppendix 5 and eFigure 2. We then created a mask that excluded from subsequent analyses any voxel in which there were baseline differences in PCC connectivity between the duloxetine and placebo arms. That is, we limited our analyses of treatment × time interactions to voxels in which baseline connectivity measures were comparable across the 2 treatment arms. The same statistical models were used for the network analyses (eAppendix 6 and eTable 4).
EXPLORATORY ANALYSIS
In the baseline scans, we examined the relationship between altered DMN connectivity and clinical symptoms in participants with DD. We calculated Pearson correlations between (1) voxelwise PCC connectivity, using whole-brain analysis, and (2) symptoms of depression as measured by the 24-item HAM-D summary score. This approach identified a relationship in the baseline MRI scans between PCC–amygdala connectivity and depressive symptom severity. We then tested whether this association persisted following treatment by calculating Pearson correlations between PCC–amygdala connectivity and posttreatment depressive symptoms.
STATISTICAL THRESHOLD
For the voxelwise whole-brain connectivity analyses, we determined an appropriate statistical threshold to account for multiple statistical comparisons by conducting Monte Carlo simulations. To achieve a corrected P < .05, we used a conjoint requirement of 100 continuous voxels, with each voxel meeting an α of less than 0.01.
RESULTS
CLINICAL TRIAL
The complete results of the clinical trial are presented elsewhere.22 Here we present the results for the participants who completed the clinical trial and MRI scans (N = 32). Of the 41 patients with DD who completed the initial MRI scan, 9 dropped out of the clinical trial prior to the follow-up MRI scan. The patients who dropped out of the clinical trial were demographically comparable with those who completed the trial (eAppendix 7). The mean (SD) dose of duloxetine at the end of the clinical trial was 91.0 (30.2) mg daily (range, 30–120 mg). We found a significant treatment × time interaction (F1,29 = 17.6; P < .001; η2 = 0.38), with the duloxetine arm demonstrating a greater reduction in depressive symptoms than the placebo arm. Specifically, the mean (SD) HAM-D summary score declined more in the duloxetine arm (pretreatment, 20.0 [0.9]; posttreatment, 5.8 [1.6]) than in the placebo arm (pretreatment, 21.4 [0.8]; posttreatment, 17.3 [1.5]).
BASELINE SCANS
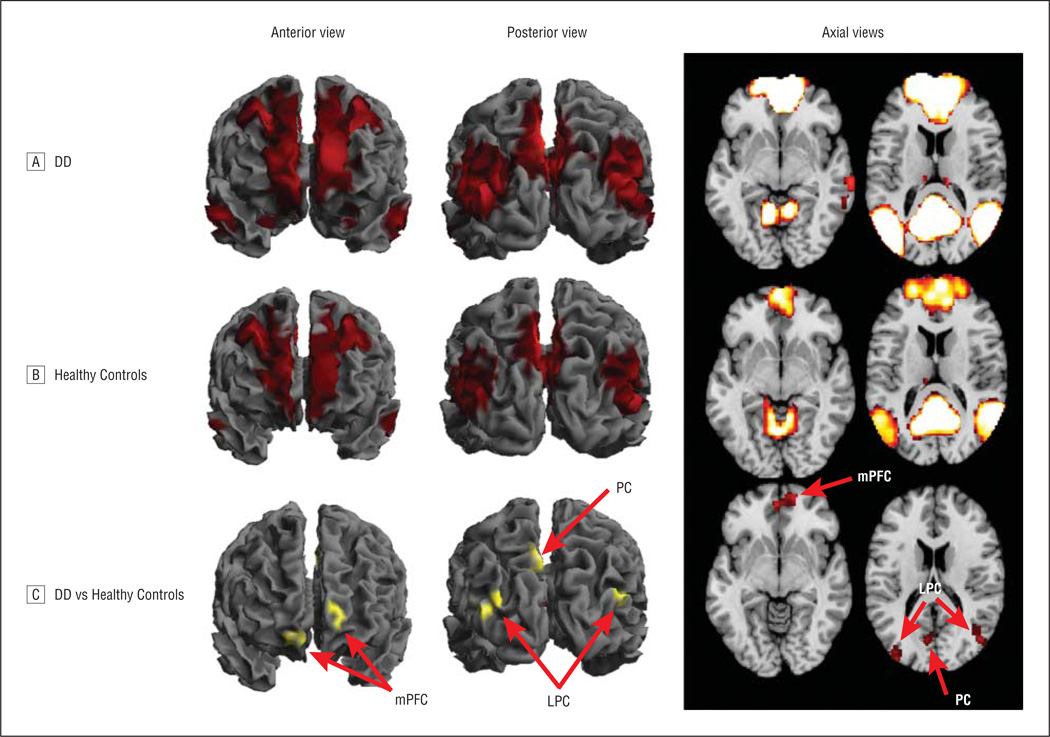
We generated voxelwise, whole-brain maps of RS-FC generated from a seed region in the PCC. Resting-state connectivity maps demonstrated the commonly observed connectivity pattern of the DMN in both participant groups (Figure 1). In both groups, significant connectivity of the PCC was detected with the precuneus, mesial prefrontal cortex, lateral parietal cortex bilaterally, superior frontal cortex bilaterally, parahippocampal gyrus bilaterally, and retrosplenial cortex.
Figure 1.
Whole-brain resting-state functional-connectivity maps with seed region in the posterior cingulate cortex. Qualitatively, the connectivity maps demonstrate the commonly observed connectivity pattern of the default mode network in both the participants with dysthymic disorder (DD; N = 41) (A) and in the healthy control participants (N = 25) (B). C, Comparison of the 2 groups demonstrated that the participants with DD had stronger connections from the posterior cingulate cortex to the mesial prefrontal cortex (mPFC) bilaterally, lateral parietal lobes (LPC) bilaterally, and precuneus (PC). See Table 2 for statistical significance.
Hypothesis Testing
Comparison of the 2 groups (Figure 1C) using a random-effects, factorial model demonstrated that compared with HC subjects, patients with DD had stronger connections between the PCC and the mesial prefrontal cortex bilaterally, lateral parietal lobes bilaterally, and precuneus (Table 2). Controlling for substance abuse and/or prior medication exposure did not meaningfully influence these results (eTable 5). After controlling for current or prior comorbid anxiety disorders and/or prior episodes of MDD, the statistically significant differences in DMN connectivity between patients with DD and HC participants were still present. However, these covariates produced a consistent pattern in which the magnitude of the statistical difference between the 2 groups (ie, DD vs HC) was reduced (eTable 5).
Table 2.
Functional Connectivity in the Default Mode Network of Patients With Dysthymic Disorder vs Healthy Control Participants
| Connection Strength With Seed in the Posterior Cingulate Cortex |
MNI Coordinate | Cluster Size | T Statistic | P Value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Connection Strength Greater in Dysthymic Participants vs Control Subjects | ||||||
| Superior frontal cortex/mesial prefrontal cortex | ||||||
| Right | 12 | 60 | −8 | 193 | 2.95 | .002 |
| Left | −14 | 66 | 4 | 103 | 3.08 | .001 |
| Lateral parietal cortex | ||||||
| Left | −36 | −90 | 22 | 266 | 3.20 | .001 |
| Right | 48 | −72 | 18 | 218 | 3.09 | .001 |
| Precuneus | −8 | −70 | 48 | 448 | 3.53 | <.001 |
| Connection Strength Greater in Control Subjects vs Dysthymic Participants | ||||||
| None detected | ||||||
Abbreviation: MNI, Montreal Neurological Institute.
Network Analysis
To confirm the observation of greater DMN connectivity in patients with DD vs HC participants, we used graph analysis to quantify the density of DMN connections. Graph analysis demonstrated that compared with HC subjects, participants with DD had greater DMN connection density (mean DMN density: participants with DD, 0.23, and HC subjects, 0.17; t = 2.7; P = .01) (Figure 2; see eAppendix 4 for nonvalued graph analysis). Using bootstrapping, we confirmed that the network findings were not driven by differences between the sample sizes (DD: N = 41 vs HC: N = 25) (eAppendix 8). Controlling for prior episodes of MDD, substance abuse, and/or prior medication exposure did not meaningfully influence these results (eTable 6). Similar to the voxelwise analysis, inclusion of current or prior comorbid anxiety disorders as a covariate yielded results that remained statistically significant, but the magnitude of the statistical difference was reduced (eTable 6).
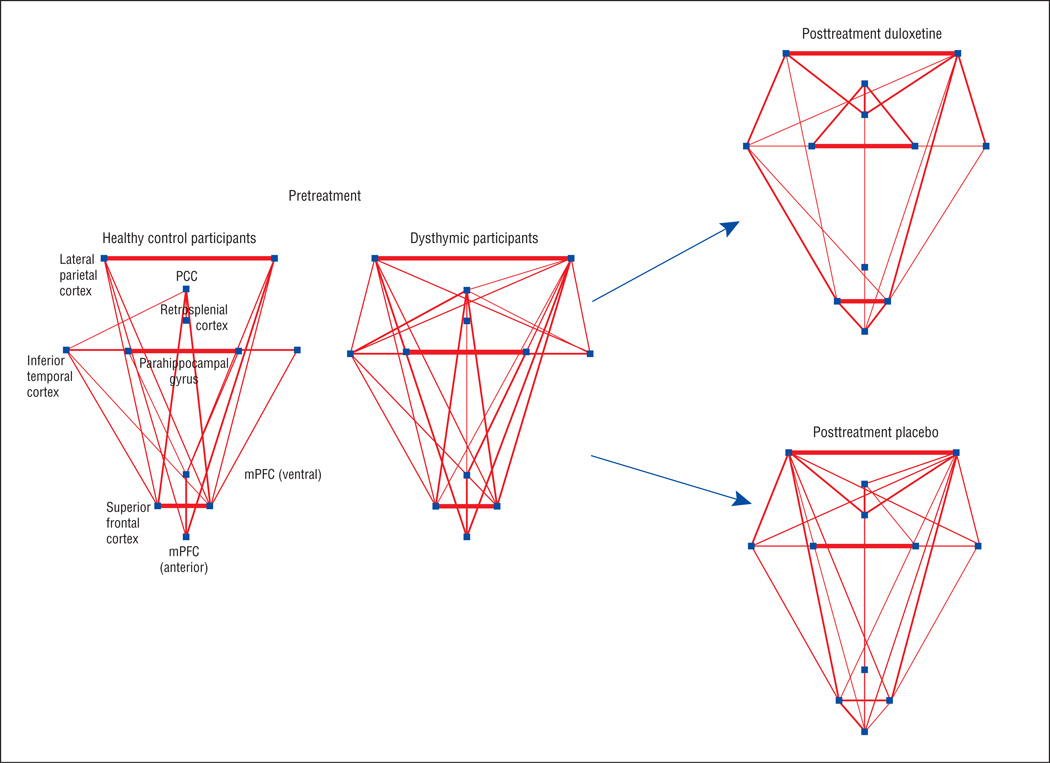
Figure 2.
Graphical presentation of the default mode network (DMN). Pretreatment magnetic resonance imaging scans were obtained in participants with dysthymic disorder (N = 41) and matched healthy control subjects (N = 25). Posttreatment magnetic resonance imaging scans were obtained following a 10-week clinical trial of duloxetine vs placebo. The dots represent DMN regions and the lines represent functional connections (threshold z >0.2); line width corresponds to the connection strength. Qualitatively, in the pretreatment scans, the DMN demonstrates more and stronger connections in the dysthymic vs healthy control participants. Statistical comparison using graph theory (network density) bears out this observation (see Table 2). Posttreatment scans demonstrate a treatment × time interaction (F1,29 = 5.0; P = .03), driven by a greater reduction in DMN connectivity in the duloxetine-treated vs placebo-treated participants (N = 15 and N = 17, respectively). PCC indicates posterior cingulate cortex.
FOLLOW-UP SCANS
Hypothesis Testing
To test our hypothesis that DMN connectivity would normalize in the duloxetine, but not placebo, arm, we examined treatment × time interactions. We began by examining the 5 regions in which we detected connectivity differences between patients with DD and HC subjects in the baseline scans (Table 2). Of these, we found a significant interaction in the right lateral parietal cortex that was driven by a decrease in PCC–right lateral parietal cortex connectivity in the duloxetine-treated arm (Table 3). Post hoc analysis of the entire DMN demonstrated interactions in the right middle to superior frontal cortex, and the right inferior temporal gyrus. We detected reductions in connectivity in the duloxetine, but not placebo, arm (Table 3). Each treatment × time interaction indicated a reduction in DMN connectivity in the duloxetine arm (ie, there were no interactions indicating an increase in DMN connectivity in the duloxetine arm). Post hoc analyses of the interactions (eAppendix 9) determined that in the duloxetine arm, the PCC–right lateral parietal cortex and PCC–right inferior temporal gyrus connections were (1) increased at baseline relative to HC subjects and (2) normalized after treatment with duloxetine (eFigure 3).
Table 3.
Connections Demonstrating Treatment × Time Interactions
| Connection Strength With Seed in the Posterior Cingulate Cortex |
MNI Coordinate | Cluster Size | Interaction Term |
Treatment Period |
Connection Strength, Mean (SD) | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Placebo | Duloxetine | ||||
| Right lateral parietal cortex | 48 | −72 | 18 | 233 | F = 7.3 | Post | 0.42 (0.2) | 0.27 (0.2) |
| P = .01 | Pre | 0.39 (0.2) | 0.54 (0.2) | |||||
| Right midsuperior frontal cortex | 42 | 14 | 48 | 274 | F = 10.0 | Post | 0.21 (0.2) | 0.02 (0.1) |
| P = .004 | Pre | 0.08 (0.2) | 0.14 (0.2) | |||||
| Right inferior temporal gyrus | 64 | −10 | −32 | 262 | F = 10.8 | Post | 0.14 (0.1) | −0.02 (0.2) |
| P = .003 | Pre | 0.13 (0.2) | 0.26 (0.1) | |||||
Abbreviation: MNI, Montreal Neurological Institute.
Network Analysis
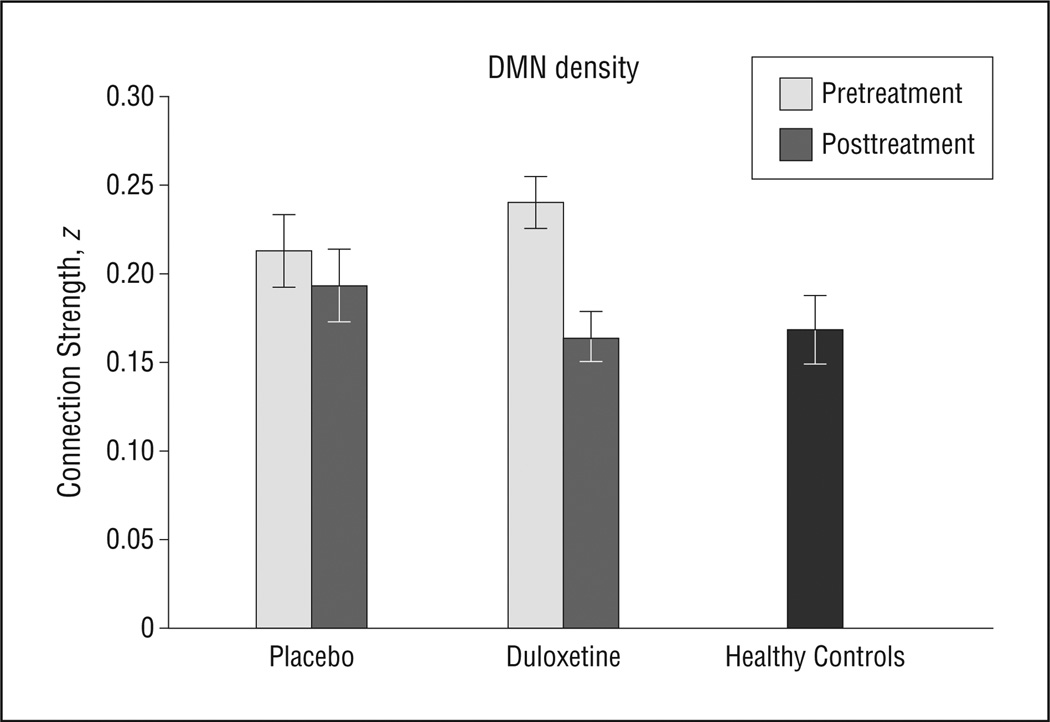
To confirm the voxelwise observation of treatment × time interactions in the DMN, we conducted a parallel analysis based on DMN connection density. Network analysis circumvents problems of multiple comparisons and related thresholding effects that are inherent to voxelwise analyses. Complementing the voxelwise analysis, the density analysis demonstrated a treatment × time interaction (Figure 2 and Figure 3; F1,29 = 5.0; P = .03). Specifically, mean DMN density in the placebo arm was similar in the baseline vs follow-up scans (Figure 2 and Figure 3; mean [SD] baseline density, 0.21 [0.08]; mean [SD] follow-up density, 0.19 [0.08]), but there was a significant reduction in the DMN density in the duloxetine arm (Figure 2 and Figure 3; mean [SD] baseline density, 0.24 [0.06]; mean [SD] follow-up density, 0.16 [0.06]; change in density: t = 4.0; P = .002). Post hoc testing demonstrated that in the duloxetine arm, the DMN density in the follow-up scans was comparable with HC subjects (t = 0.4; P = .90).Likewise, the DMN density in the follow-up scans was significantly lower in the duloxetine vs placebo arms (mean [SD] DMN density: duloxetine arm, 0.16 [0.06], and placebo arm, 0.21 [0.08]; t = 1.9; P = .04). Sensitivity analyses of the treatment × time interaction to examine the influence of potential confounds such as age and sex demonstrated that these covariates did not meaningfully influence the results (eAppendix 10).
Figure 3.
Comparison of the pretreatment and posttreatment scans demonstrated a treatment × time interaction (F1,29 = 5.0; P = .03) in the connection density of the default mode network (DMN). Specifically, mean DMN density in the placebo arm (N = 17) was similar in the baseline vs follow-up scans (mean [SD] baseline density, 0.21 [0.08]; mean [SD] follow-up density, 0.19 [0.08]), but there was a significant reduction in the DMN density in the duloxetine arm (N = 15) (mean [SD] baseline density, 0.24 [0.06]; mean [SD] follow-up density, 0.16 [0.06]; change in density: t = 4.0; P = .002). Post hoc testing demonstrated that in the duloxetine arm, the DMN density in the follow-up scans was comparable with healthy control subjects (N = 25) (t = 0.4; P = .90). The error bars indicate standard errors.
Exploratory Analysis and Clinical Correlates
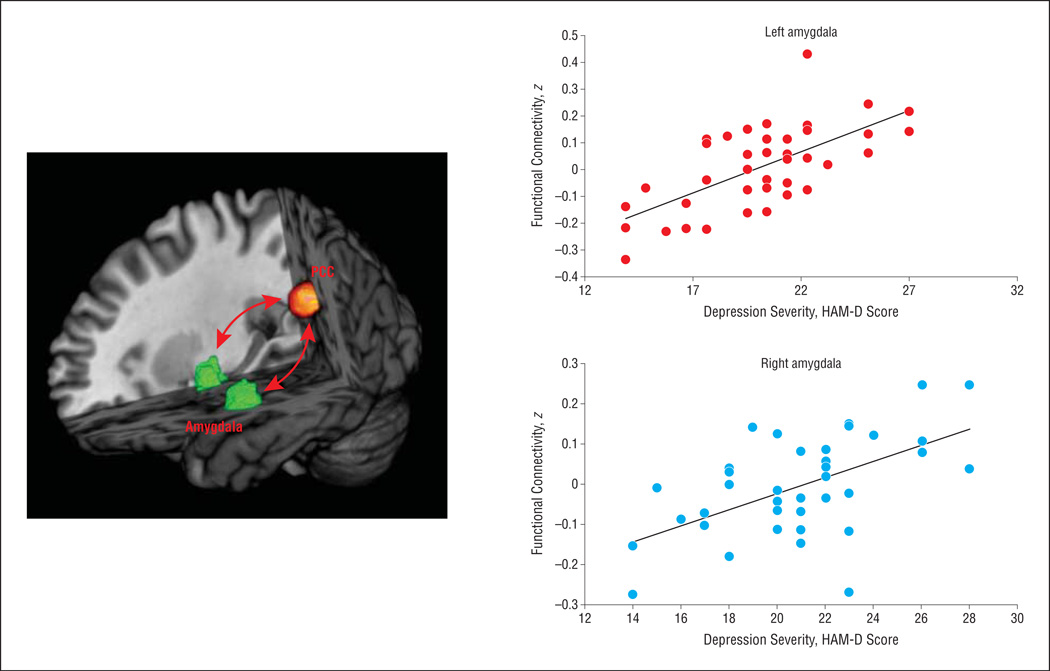
We conducted 2 exploratory analyses. In the first, we tested whether there was a relationship between the reduction in DMN connectivity and symptom improvement, as determined by the posttreatment HAM-D summary score (covarying for baseline HAM-D scores). We found no evidence for this relationship in either treatment arm. Second, we conducted a whole-brain correlation analysis of PCC connectivity with the severity of depressive symptoms within the DD group. In the baseline scans, we found that the connection strength between the PCC and the left amygdala (peak voxel Montreal Neurological Institute coordinates: x = −18, y = −4, z = −22) was a strong predictor of HAM-D scores (Figure 4; r = 0.65; P < .001; cluster size, 503 voxels). The connection strength between the PCC and the right amygdala was also a predictor of HAM-D scores (Figure 4; r = 0.58; P < .001; peak voxel Montreal Neurological Institute coordinates: x = 36, y = 0, z = −26; cluster size, 43 voxels), but this finding did not reach both arms of our statistical threshold (ie, α < 0.01, but <100 continuous voxels achieved this α level). Posterior cingulate cortex–amygdalar connectivity no longer correlated with depressive symptom severity following treatment with either placebo or duloxetine (eAppendix 11).
Figure 4.
Connection strength between the posterior cingulate cortex (PCC) and left amygdala (peak voxel Montreal Neurological Institute coordinates: x = −18, y = −4, z = −22) predicted depressive symptoms on the Hamilton Depression Rating Scale (HAM-D) (r = 0.65; P < .001; cluster size, 503 voxels) in patients with dysthymia (N = 41). The connection strength between the PCC and the right amygdala was also a predictor of HAM-D scores (r = 0.58; P < .001; peak voxel Montreal Neurological Institute coordinates: x = 36, y = 0, z = −26; cluster size, 43 voxels), but this finding did not reach both arms of our statistical threshold. Coronal slice displayed at y = −4.
COMMENT
To our knowledge, this is the first study to incorporate MRI into a randomized clinical trial of an antidepressant in patients with DD. The central finding is that increased baseline connectivity in the DMN of patients with DD is normalized by treatment with duloxetine. This normalization was specific to the duloxetine-treated patients and did not occur in patients who received placebo. Prior studies of depression have demonstrated functional changes in the prefrontal and subgenual anterior cingulate cortices following open treatment with antidepressant medication.35,36 However, these prior studies lacked placebo control, making it difficult to discern medication effects from nonspecific effects. By incorporating a placebo-controlled design, we were able to move beyond correlative interpretations and impute causation. Placebo-controlled trials permit causal interpretations of medication effects on symptom reduction; similarly, causal interpretations of medication effects on MRI findings can be imputed when the MRI findings are the outcome measure of the clinical trial.37 Thus, to our knowledge, this is the first MRI study to demonstrate a causal relationship between antidepressant use and the normalization of a specific neural anomaly in depressed patients. Recent articles in the lay press and professional journals have questioned whether antidepressants have any benefit beyond that delivered by placebo in patients with milder forms of depression.38,39 Our study provides biological evidence for a normalizing effect of antidepressants that cannot be attributed to placebo in patients with chronic, mild depression.
An important caveat to our findings is that we did not find a relationship between normalization of the DMN and a reduction in depressive symptoms. We suspected that this may be owing to the hypothesized role of the DMN in ruminative introspection. That is, the normalization of the DMN may accompany improvement in a specific symptom domain (ie, rumination), rather than the full range of depressive symptoms indexed by the HAM-D. Lacking a detailed assessment of ruminative symptoms, we cannot test this hypothesis directly, although it could be tested readily in future studies. Indeed, recent studies have shown that atypical RS DMN activity in patients with MDD correlates with behavioral measures of rumination.40,41
Duloxetine is a serotonin and norepinephrine reuptake inhibitor with balanced inhibition of both receptor systems. Therefore, inhibition of either system could, in theory, be responsible for duloxetine’s normalizing effect on DMN connectivity. However, recent studies have suggested that neural activity in the DMN is affected by the serotonin system. A recent study combined positron-emission tomography and fMRI and found that the introspective functioning of the DMN is modulated by serotonin receptor binding in the PCC and medial prefrontal cortex.18 This complements prior studies showing that PCC activity is influenced by tryptophan depletion19 and selective serotonin reuptake inhibitor administration.42 Future studies could directly test whether reuptake inhibition of serotonin, norepinephrine, or both is responsible for normalizing DMN connectivity by comparing the effects of serotonin vs norepinephrine reuptake inhibitors (eg, fluoxetine and reboxetine, respectively) on DMN connectivity.
Several additional findings are noteworthy. First, we found that at baseline, patients with DD, relative to HC subjects, had increased DMN connectivity. Recent studies have suggested that abnormal functioning of the brain’s DMN may play a significant role in the pathophysiology of depressive disorders. For example, in an fMRI study of the DMN in 24 adults with MDD, the depressed participants, unlike HC subjects, did not suppress DMN activity when viewing negatively valenced pictures or when instructed to reappraise the pictures.17 Likewise, atypical RS connectivity between the DMN and the subgenual cingulate cortex is reported in adults with MDD.7 To our knowledge, our study is the first to examine connectivity of the DMN in persons with DD, and it suggests that DD and MDD share pathophysiological features, at least in terms of abnormal DMN connectivity. Thus, this study adds neurobiological support for the idea of merging DD with other chronic depressions, as discussed by the DSM-5 workgroup.43
In the baseline MRI scans, we found that DMN connectivity was altered in patients with DD regardless of whether the patients had a history of MDD, diminishing the possibility that altered DMN connectivity in patients with DD was merely a consequence of prior episodes of MDD. Investigators have argued that DD can be conceptualized as a prodrome of MDD, given that DD strongly predicts the development of MDD, whereas MDD does not predict the development of DD.44 Together with the findings that DMN connectivity is altered in both DD and MDD, this consideration leads us to speculate that increased DMN connectivity may convey vulnerability for developing MDD. A longitudinal neuroimaging study could test this hypothesis and, more importantly, could help determine whether normalizing altered DMN connectivity through pharmacologic or psychotherapeutic interventions affects the course of illness.
Another contribution that our study makes to the literature on depression is the strong correlation between depressive symptoms and the strength of functional connections between the PCC and the amygdala. A similar finding has been reported in generalized anxiety disorder,45 a condition highly comorbid with DD.46 The amygdala is more strongly associated with processing information that is negatively vs positively valenced,47 and it tends to be hyperactive in depressed patients.48 We suspect that increased connectivity between the amygdala and the PCC (and by extension the DMN) contributes to more severe depressive symptoms because of the putative role of the DMN in ruminative introspection. Our findings suggest that as connectivity between the amygdala and the DMN increases, the DMN’s information processing functions may become increasingly tinged with negative cognitions. Posttreatment, we found that a relationship between amygdalar-DMN connectivity and depressive symptom severity was no longer significant, suggesting that this relationship is altered by treatment with either duloxetine or placebo.
Limitations of this study should be noted. First, many of the patients with DD had prior exposure to treatment with psychotropics. Therefore, the differential connectivity that we detected may have been the product of prior medication exposure rather than DD itself. We attempted to minimize this possibility by (1) statistically controlling for prior medication exposure and (2) obtaining MRI scans after a medication washout period. Nevertheless, we cannot entirely exclude the possibility that medication effects influenced our findings. Second, comorbid anxiety disorders and prior depressive episodes were present in many of the participants with DD. Covarying for anxiety and/or prior depressive episodes produced a consistent pattern in which the difference in connectivity between subjects with DD and HC participants remained statistically significant, but the magnitude of the difference was reduced. This suggests that anxiety and prior depressive episodes may contribute to the increased DMN connectivity that we detected. Third, our cohort of participants with DD consisted of more men than women (59% men). This sex distribution could limit the generalizability of our findings given that depressive disorders, including DD, affect women in greater numbers than men. Fourth, although we attempted to exclude from the study patients with a diagnosis of MDD, differentiating DD and MDD can be difficult, thus it is possible that some of the DD cohort may have met criteria for MDD. Fifth, we did not examine whether the HC participants had a genetic vulnerability to depression. A control sample absent of genetic loading for depression could potentially have yielded more robust group differences. Finally, a larger cohort could help establish the stability of our findings.
In conclusion, our study demonstrates the atypical nature of DMN connectivity in adults with DD and provides important neurobiological evidence for a causal mechanism by which antidepressant therapy normalizes the brains of depressed patients. Altered DMN connectivity in persons with DD was detected irrespective of prior episodes of MDD, suggesting that altered DMN connectivity is shared between DD and MDD, as well as providing neurobiological support for a contemplated nosologic revision to merge DD with other forms of chronic depression. Finally, increased connection strength between the amygdala and the PCC may represent an important target for novel antidepressant therapeutics.
Acknowledgments
Funding/Support: This study was supported in part by National Institute of Mental Health grants K23-MH091249 (Dr Posner) and K02-74677 (Dr Peterson), as well as funding from the Brain and Behavior Research Foundation (formerly the National Alliance for Research in Schizophrenia and Affective Disorders) and Eli Lilly and Company.
Additional Contributions: We thank Satie Shova, BSRT, for her technical assistance and Jun Liu, PhD, for his assistance with the statistical analyses.
Footnotes
Author Contributions: Dr Posner had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: Dr Posner is a principal investigator on an investigator-initiated grant from Shire Pharmaceuticals. Drs Hellerstein and Peterson are coprincipal investigators on an investigator-initiated grant from Eli Lilly and Company. Dr Hellerstein is a principal investigator on an investigator-initiated grant from Pfizer Pharmaceuticals. Dr McGrath has received research support from F. Hoffman-LaRoche Ltd. Dr Stewart has received research support from Pfizer, Alkermes, Forest Pharmaceuticals, and Shire Pharmaceuticals.
Online-Only Material: The eAppendices, eFigures, and eTables are available at http://www.jamapsych.com.
REFERENCES
- 1.Weissman MM, Leaf PJ, Bruce ML, Florio L. The epidemiology of dysthymia in five communities: rates, risks, comorbidity, and treatment. Am J Psychiatry. 1988;145(7):815–819. doi: 10.1176/ajp.145.7.815. [DOI] [PubMed] [Google Scholar]
- 2.Howland RH. General health, health care utilization, and medical comorbidity in dysthymia. Int J Psychiatry Med. 1993;23(3):211–238. doi: 10.2190/AXCU-P704-23XQ-CQTR. [DOI] [PubMed] [Google Scholar]
- 3.Hellerstein DJ, Kocsis JH, Chapman D, Stewart JW, Harrison W. Double-blind comparison of sertraline, imipramine, and placebo in the treatment of dysthymia: effects on personality. Am J Psychiatry. 2000;157(9):1436–1444. doi: 10.1176/appi.ajp.157.9.1436. [DOI] [PubMed] [Google Scholar]
- 4.Ravindran AV, Smith A, Cameron C, Bhatla R, Cameron I, Georgescu TM, Hogan MJ. Toward a functional neuroanatomy of dysthymia: a functional magnetic resonance imaging study. J Affect Disord. 2009;119(1–3):9–15. doi: 10.1016/j.jad.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Tebartz van Elst L, Woermann FG, Lemieux L, Trimble MR. Amygdala enlargement in dysthymia: a volumetric study of patients with temporal lobe epilepsy. Biol Psychiatry. 1999;46(12):1614–1623. doi: 10.1016/s0006-3223(99)00212-7. [DOI] [PubMed] [Google Scholar]
- 6.Lyoo IK, Kwon JS, Lee SJ, Han MH, Chang CG, Seo CS, Lee SI, Renshaw PF. Decrease in genu of the corpus callosum in medication-naïve, early-onset dysthymia and depressive personality disorder. Biol Psychiatry. 2002;52(12):1134–1143. doi: 10.1016/s0006-3223(02)01436-1. [DOI] [PubMed] [Google Scholar]
- 7.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117(1–2):1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161(4):598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 10.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 11.Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med. 1998;49(1):341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- 12.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- 13.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 14.Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109(3):504–511. [PubMed] [Google Scholar]
- 15.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspect Psychol Sci. 2008;3(5):400. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 16.Spasojević J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1(1):25–37. doi: 10.1037/1528-3542.1.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn A, Wadsak W, Windischberger C, Baldinger P, Höflich AS, Losak J, Nics L, Philippe C, Kranz GS, Kraus C, Mitterhauser M, Karanikas G, Kasper S, Lanzenberger R. Differential modulation of the default mode network via serotonin-1A receptors. Proc Natl Acad Sci U S A. 2012;109(7):2619–2624. doi: 10.1073/pnas.1117104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunisato Y, Okamoto Y, Okada G, Aoyama S, Demoto Y, Munakata A, Nomura M, Onoda K, Yamawaki S. Modulation of default-mode network activity by acute tryptophan depletion is associated with mood change: a resting state functional magnetic resonance imaging study. Neurosci Res. 2010;69(2):129–134. doi: 10.1016/j.neures.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) New York, New York: Biometrics Research; 1995. [Google Scholar]
- 21.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hellerstein DJ, Stewart JW, McGrath PJ, Deliyannides DA, Batchelder ST, Black SR, Withers A, O’Shea D, Chen Y. A randomized controlled trial of duloxetine versus placebo in the treatment of nonmajor chronic depression. J Clin Psychiatry. 2012;73(7):984–991. doi: 10.4088/JCP.11m07230. [DOI] [PubMed] [Google Scholar]
- 23.Friston KJ, Ashburner CD, Frith JB, Poline JB, Heather RS, Frackowiak RS. Spatial registration and normalization of images. Hum Brain Mapp. 1995;3(3):165–189. [Google Scholar]
- 24.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68(12):1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 27.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sporns O. Graph theory methods for the analysis of neural connectivity patterns. In: Kötter R, editor. Neuroscience Databases: A Practical Guide. Boston, MA: Kluwer Academic Publishers; 2002. pp. 171–186. [Google Scholar]
- 30.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott J. Social Network Analysis: A Handbook. London, England: Sage Publications; 2007. [Google Scholar]
- 33.Borgatti S, Everett M, Freeman L. Ucinet for Windows: Software for Social Network Analysis. Harvard, MA: Analytic Technologies; 2002. [Google Scholar]
- 34.Butts CT. Social network analysis: a methodological introduction. Asian J Soc Psychol. 2008;11(1):13–41. [Google Scholar]
- 35.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48(8):830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 36.Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, Sheline YI. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord. 2009;112(1–3):206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson BS. Conceptual, methodological, and statistical challenges in brain imaging studies of developmentally based psychopathologies. Dev Psychopathol. 2003;15(3):811–832. doi: 10.1017/s0954579403000385. [DOI] [PubMed] [Google Scholar]
- 38.Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. NEngl J Med. 2008;358(3):252–260. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 39.Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthews SC, Simmons AN, Strigo IA, Arce E, Stein MB, Paulus MP. Escitalopram attenuates posterior cingulate activity during self-evaluation in healthy volunteers. Psychiatry Res. 2010;182(2):81–87. doi: 10.1016/j.pscychresns.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remick RA, Sadovnick AD, Lam RW, Zis AP, Yee IM. Major depression, minor depression, and double depression: are they distinct clinical entities? Am J Med Genet. 1996;67(4):347–353. doi: 10.1002/(SICI)1096-8628(19960726)67:4<347::AID-AJMG6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 44.Lewinsohn PM, Rohde P, Seeley JR, Hops H. Comorbidity of unipolar depression, I: major depression with dysthymia. J Abnorm Psychol. 1991;100(2):205–213. [PubMed] [Google Scholar]
- 45.Strawn J, Bitter S, Cerullo M, Whitsel R, Weber W, Eliassen J, Adler C, Strakowski S, DelBello M. Neurocircuitry of generalized anxiety disorder in adolescents: a functional connectivity study. Paper presented at: 2011 American Academy of Child and Adolescent Psychiatry Annual Meeting; October 2011; Toronto, Canada. [Google Scholar]
- 46.Wittchen HU, Zhao S, Kessler RC, Eaton WW. DSM-III-R generalized anxiety disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(5):355–364. doi: 10.1001/archpsyc.1994.03950050015002. [DOI] [PubMed] [Google Scholar]
- 47.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 48.Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JDE, Gotlib IH. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16(12):1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]