Abstract
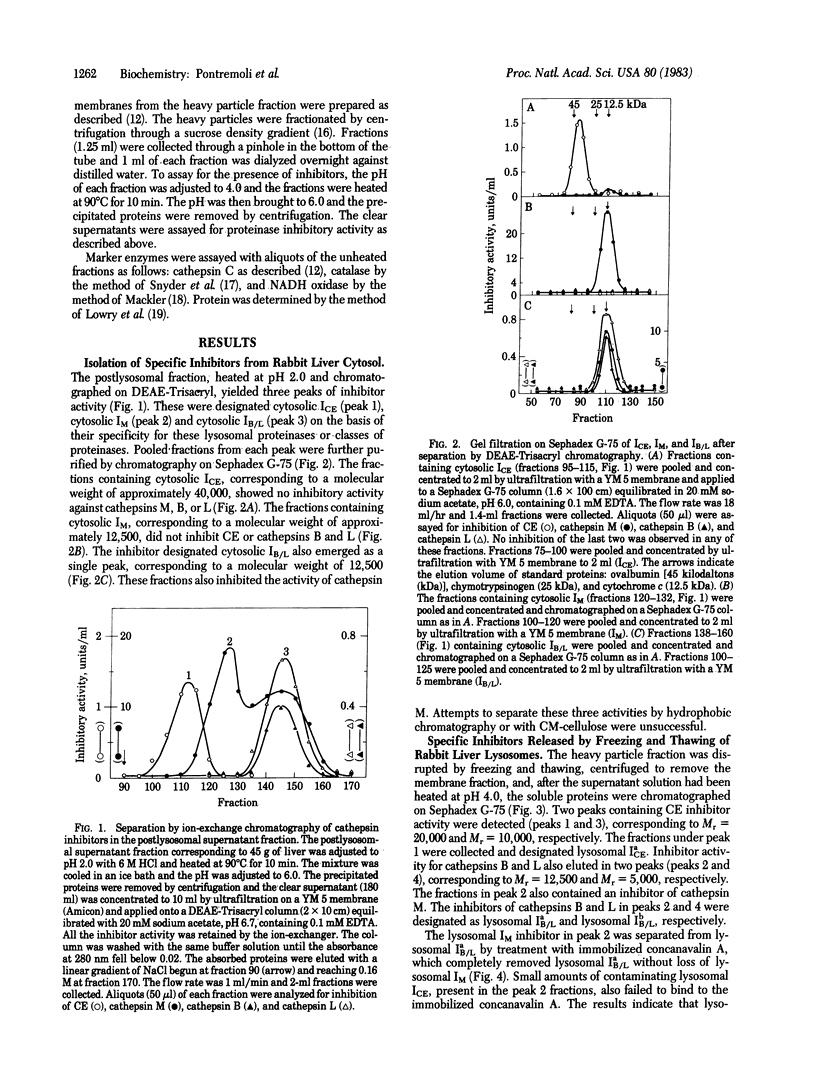
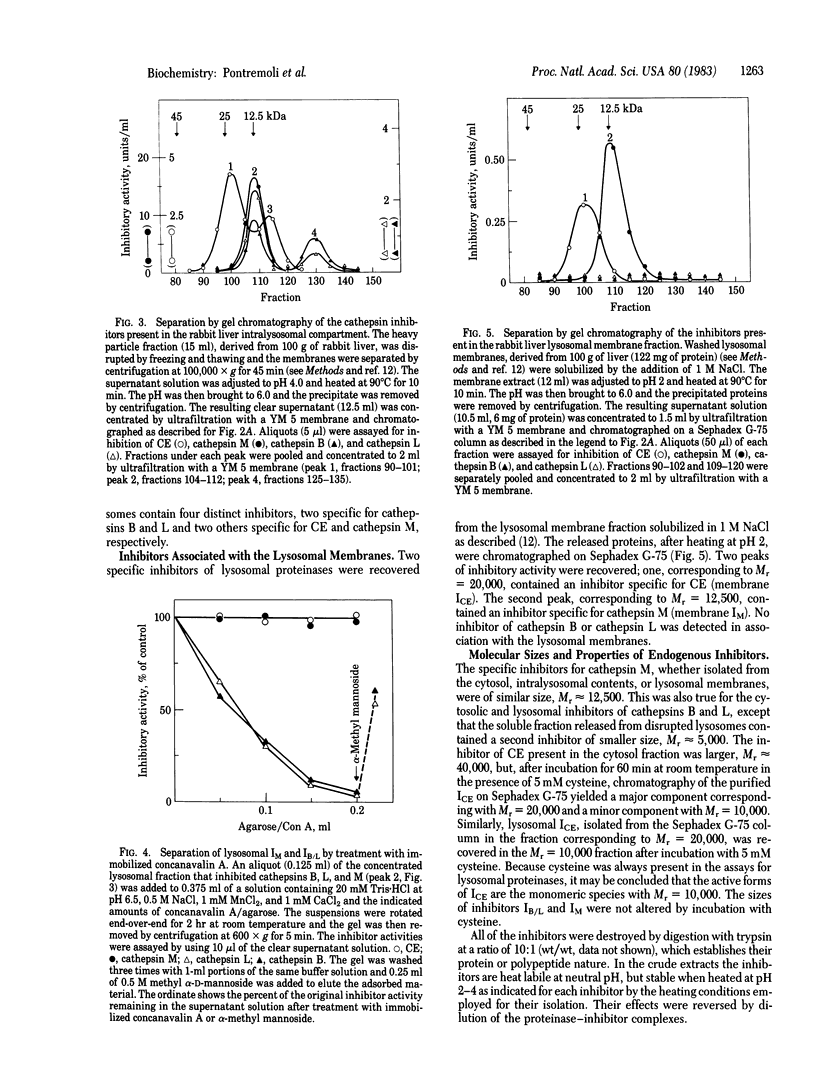
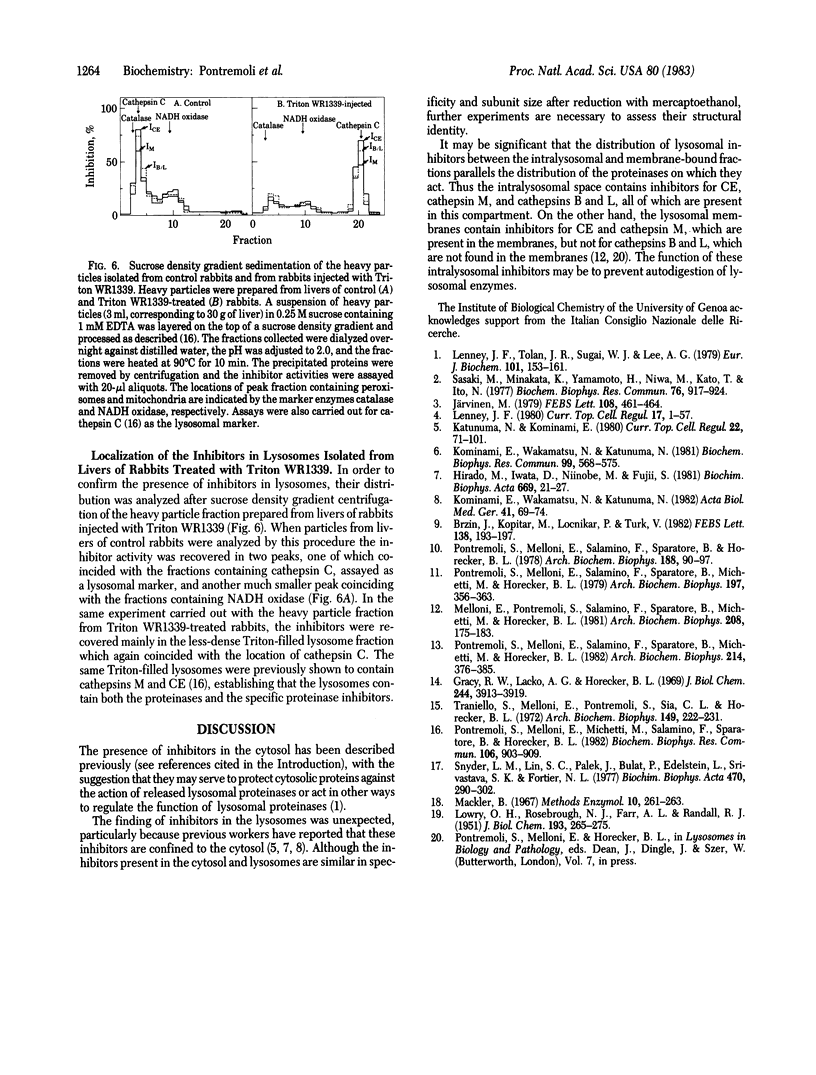
Specific inhibitors of three lysosomal proteinases are present in the cytosolic and lysosomal compartments of rabbit liver. The cytosolic inhibitors, purified by chromatography on DEAE-Trisacryl and Sephadex G-75, show specificities toward cathepsin M, cathepsins B and L, and fructose 1,6-bisphosphatase converting enzyme (CE), respectively, and are designated IM, IB/L, and ICE. Inhibitors with similar specificities have been isolated from the intralysosomal compartment. Two of these inhibitors, IM and ICE, are also present in the lysosomal membranes. The lysosomal distribution parallels that of the respective proteinases. The inhibitors are polypeptides with molecular weights of 5,000-10,000 for the two forms of IB/L, 12,500 for IM, and 10,000-40,000 for the ICE species.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brzin J., Kopitar M., Locnikar P., Turk V. An endogenous inhibitor of cysteine and serine proteinases from spleen. FEBS Lett. 1982 Feb 22;138(2):193–197. doi: 10.1016/0014-5793(82)80439-0. [DOI] [PubMed] [Google Scholar]
- Gracy R. W., Lacko A. G., Horecker B. L. Subunit structure and chemical properties of rabbit liver aldolase. J Biol Chem. 1969 Jul 25;244(14):3913–3919. [PubMed] [Google Scholar]
- Hirado M., Iwata D., Niinobe M., Fujii S. Purification and properties of thiol protease inhibitor from rat liver cytosol. Biochim Biophys Acta. 1981 Jun 29;669(1):21–27. doi: 10.1016/0005-2795(81)90218-x. [DOI] [PubMed] [Google Scholar]
- Järvinen M. Purification and some characteristics of two human serum proteins inhibiting papain and other thiol proteinases. FEBS Lett. 1979 Dec 15;108(2):461–464. doi: 10.1016/0014-5793(79)80588-8. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Kominami E. Structures and functions of lysosomal thiol proteinases and their endogenous inhibitor. Curr Top Cell Regul. 1983;22:71–101. doi: 10.1016/b978-0-12-152822-5.50007-5. [DOI] [PubMed] [Google Scholar]
- Kominami E., Wakamatsu N., Katunuma N. Endogenous thiol protease inhibitor from rat liver. Biochem Biophys Res Commun. 1981 Mar 31;99(2):568–575. doi: 10.1016/0006-291x(81)91783-6. [DOI] [PubMed] [Google Scholar]
- Kominami E., Wakamatsu N., Katunuma N. Endogenous thiol proteinase inhibitor from rat liver. Acta Biol Med Ger. 1982;41(1):69–74. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenney J. F. Inhibitors associated with the proteinases of mammalian cells and tissues. Curr Top Cell Regul. 1980;17:25–57. doi: 10.1016/b978-0-12-152817-1.50006-7. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Tolan J. R., Sugai W. J., Lee A. G. Thermostable endogenous inhibitors of cathepsins B and H. Eur J Biochem. 1979 Nov 1;101(1):153–161. doi: 10.1111/j.1432-1033.1979.tb04227.x. [DOI] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Salamino F., Sparatore B., Michetti M., Horecker B. L. Characterization of three rabbit liver lysosomal proteinases with fructose 1,6-bisphosphatase converting enzyme activity. Arch Biochem Biophys. 1981 Apr 15;208(1):175–183. doi: 10.1016/0003-9861(81)90137-5. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Michetti M., Salamino F., Sparatore B., Horecker B. L. Localization of two lysosomal proteinases on the external surface of the lysosomal membrane. Biochem Biophys Res Commun. 1982 Jun 15;106(3):903–909. doi: 10.1016/0006-291x(82)91796-x. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., Sparatore B., Horecker B. L. Interactions of Zn2+ and Mg2+ with rabbit liver fructose 1,6 bisphosphatase. Arch Biochem Biophys. 1978 May;188(1):90–97. doi: 10.1016/0003-9861(78)90360-0. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., Sparatore B., Michetti M., Horecker B. L. Cathepsin M: a lysosomal proteinase with aldolase-inactivating activity. Arch Biochem Biophys. 1982 Mar;214(1):376–385. doi: 10.1016/0003-9861(82)90042-x. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., Sparatore B., Michetti M., Singh V. N., Horecker B. L. Evidence for an interaction between fructose 1,6-bisphosphatase and fructose 1,6-bisphosphate aldolase. Arch Biochem Biophys. 1979 Oct 1;197(1):356–363. doi: 10.1016/0003-9861(79)90256-x. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Minakata K., Yamamoto H., Niwa M., Kato T., Ito N. A new serum component which specifically inhibits thiol proteinases. Biochem Biophys Res Commun. 1977 Jun 6;76(3):917–924. doi: 10.1016/0006-291x(77)91589-3. [DOI] [PubMed] [Google Scholar]
- Snyder L. M., Liu S. C., Palek J., Bulat P., Edelstein L., Srivastava S. K., Fortier N. L. Partition of catalase and its peroxidase activities in human red cell membrane: effect of ATP depletion. Biochim Biophys Acta. 1977 Oct 17;470(2):290–302. doi: 10.1016/0005-2736(77)90107-9. [DOI] [PubMed] [Google Scholar]
- Traniello S., Melloni E., Pontremoli S., Sia C. L., Horecker R. L. Rabbit liver fructose 1,6-diphosphatase. Properties of the native enzyme and their modification by subtilisin. Arch Biochem Biophys. 1972 Mar;149(1):222–231. doi: 10.1016/0003-9861(72)90317-7. [DOI] [PubMed] [Google Scholar]


