Figure 4. Structures of sagEsxA and CFP-10/ESAT-6 complexes, and comparisons of the loop conformation, as observed in the three WXG100 proteins.
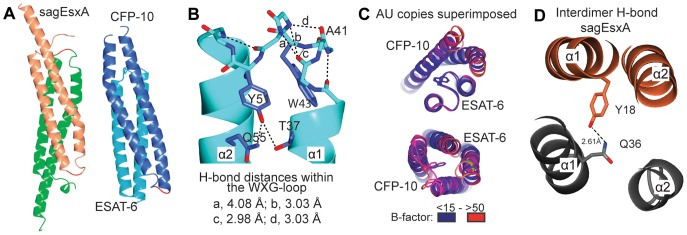
(A) The four-helix bundle structures of the homodimeric sagEsxA and heterodimeric CFP-10/ESAT-6 complexes are shown. (B) The WXG motif-containing loops of ESAT-6 showing an extended hydrogen-bonding network as indicated by dashed lines and labelled with their hydrogen bond donor-acceptor distances. (C) Comparisons of the loops of CFP-10 and ESAT-6. The asymmetric unit (AU) of CFP-10/ESAT-6 crystal contains two copies of the heterodimer. The view shows down towards the central long axis of the dimer. The relation of top to bottom panel views are 180° rotation around central short axis of the dimer, showing the WXG containing loop of ESAT-6 (top) and that of CFP-10 (bottom). Superimpositions of the structures of the AU content show that the WXG containing loops of ESAT-6 exhibit lower B-values and overlap better than that of CFP-10. (D) A hydrogen bond interaction formed by Y18 and Q38 at the inter-dimer interface of sagEsxA is shown.
