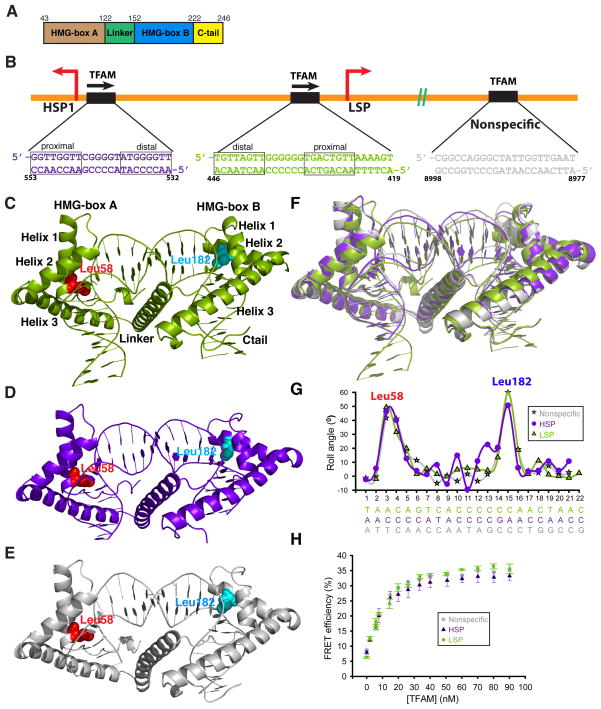
Figure 1. Overview of the TFAM-mtDNA complexes.
(A) The domain structure of mature TFAM. Residues 1–42 constitute the mitochondrial targeting sequence that is cleaved upon import of TFAM into the mitochondrial matrix. (B) Schematic of DNA sequences bound within TFAM crystals. Note the different orientations of TFAM on LSP versus HSP1. The nonspecific sequence is from the ATPase6 gene. The half-sites of LSP and HSP1 are indicated. (C), (D), (E) Side view of the TFAM/LSP, TFAM/HSP1, and TFAM/nonspecific DNA complexes, respectively. The major intercalating residues, Leu58 and Leu182, are highlighted. The DNA fragments are color-coded as in (B). (F) Superimposition of TFAM crystal structures, color-coded as in (B). (G) Comparison of roll angle values for TFAM/LSP, TFAM/HSP1, and TFAM/nonspecific DNA. Note that there are two peaks of DNA distortion, at the positions where Leu58 and Leu182 intercalate. (H) FRET assay for DNA bending with three different DNA templates: LSP, HSP1, and nonspecific DNA. Data points are the average of three independent experiments, with error bars representing standard deviations.