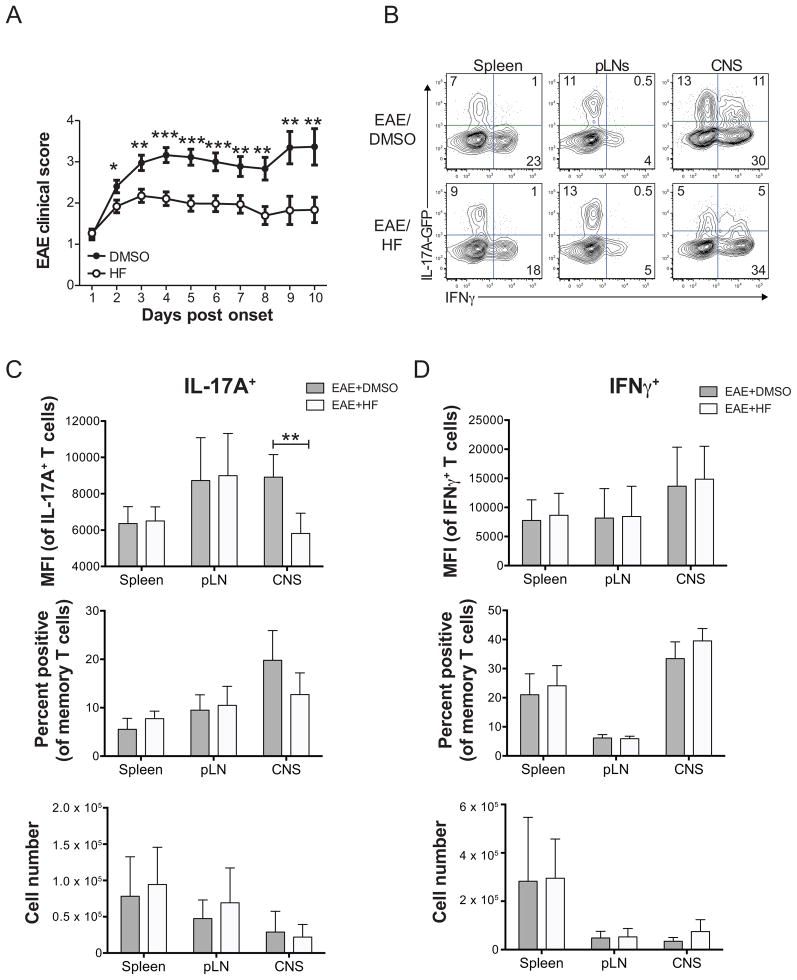
Figure 4. HF treatment reduces established EAE disease and inhibits Th17 effector function in the CNS. (A).
Mean clinical EAE score ± SD in wild-type C57BL/6 mice immunized with MOG33–55 and treated daily with HF (0.3 mg/kg; n = 43) or vehicle (DMSO; n = 40) beginning at onset of disease symptoms. * P < .05; ** P < .01; *** P < .001 by paired student’s t test. (B) Cytokine expression in ex vivo-isolated and PMA+ionomycin-stimulated mononuclear cells from the spleen, peripheral lymph nodes (pLNs), or brain and spinal cord (CNS) of vehicle- (EAE score = 3) or HF- (EAE score = 2) treated mice with active EAE on day 6 post disease onset/HF treatment. Data shown are on CD45+TCRβ+CD4+CD44hi-gated memory T cells. Data represent 2 independent experiments, with each including analysis of 3 mice per group. Analysis of IL-17A (C) or IFNγ (D) expression by CD4+CD44hi effector/memory T cells from spleen, pLNs, or brain and spinal cord (CNS) of 6 DMSO- or HF-treated IL-17A-GFP reporter mice with active EAE on day 6 post disease onset/HF treatment. Top – mean fluorescence intensity of IL-17A (GFP) or IFNγ (intracellular cytokine staining) expression in effector/memory cells gated as positive for cytokine expression. Middle – percentage of effector/memory cells from each organ displaying detectable IL-17A or IFNγ expression. Bottom – absolute numbers of IL-17A+ or IFNγ+ effector/memory T cells, calculated as follows: total number of mononuclear cells recovered x % of live lymphocytes x % of CD4+CD44hi effector/memory T cells x % of cytokine positive cells. All data are mean values ± SD from 6 mice per group across two independent experiments. ** P < .01 by paired student’s t test.