Abstract
Objective
To determine the rates and causes of first antiretroviral treatment (ART) changes in HIV-infected adults in Côte d'Ivoire.
Methods
We evaluated adults who initiated ART in an outpatient clinic in Abidjan. We recorded baseline and follow-up data, including drug prescriptions and reasons for changing to alternative first-line regimens (drug substitution for any reason but failure) or second-line regimens (switch for failure).
Results
2012 HIV-infected adults (73% women) initiated ART. At baseline, 9% of all patients were on treatment for tuberculosis and 3% of women were pregnant. First-line ART consisted of 2 NRTIs (58% stavudine-lamivudine, 42% zidovudine-lamivudine) and efavirenz (63%), nevirapine (32%) or indinavir (5%). Median follow-up time was 16.9 months. During this time, 205 (10%) patients died and 261 (13%) were lost to follow-up. Overall, the rate of treatment modifications was 20.7/100 patient-years (PY). The most common modifications were drug substitutions for intolerance (12.4/100PY), pregnancy (4.5/100PY) and tuberculosis (2.5/100PY). The rates of intolerance-related substitutions were 17.9/100PY for stavudine, 6.3/100PY for nevirapine, 3.9/100PY for zidovudine and 0.1/100PY for efavirenz. Twenty percent of efavirenz substitutions resulted from pregnancy and 18% of nevirapine substitutions were related to tuberculosis treatment.
Conclusions
During the first months following ART initiation, a third of all treatment changes occurred for reasons other than intolerance to the drug or treatment failure. In Africa, drug forecasting is crucial to ensuring the success of HIV treatment programs. Drugs that do not require interruptions during pregnancy or tuberculosis treatment should be made more readily available as first-line drugs in sub-Saharan Africa.
Keywords: adults, sub-Saharan Africa, antiretroviral treatment, modification, tolerance, pregnancy, tuberculosis, efavirenz, nevirapine
Introduction
In 2007, the World Health Organization (WHO) estimated that 480,000 adults and children were living with HIV in Côte d'Ivoire. Of these HIV-infected individuals, 190,000 persons were estimated to be in need of antiretroviral treatment (ART), of whom only 51,812 (28%) were actually receiving ART [1][2]. As the number of patients starting ART in Côte d'Ivoire continues to rise, numerous medical and logistical challenges have arisen, at both the individual and national levels.
In order for an HIV-treatment program to be successful, appropriate drugs need to be available in sufficient quantity. Three indicators are essential for the maintenance of drug supplies: rate of treatment initiation among ART-naïve patients, rate of retention in care and rate of treatment modifications. Although in recent years an increasing number of publications have reported on the number of patients starting ART and remaining in care in large sub-Saharan African programs [3-7], reports on the rates of ART regimen modifications and their causes have remained scarce [8, 9].
Here we report the frequency and causes of first-time ART modifications in a cohort of HIV-infected adults who were followed up for 2.5 years after starting ART in a large HIV care clinic in Abidjan, Côte d'Ivoire.
Methods
Setting and Patients
In 2003, health professionals from the ANRS/Ministry of Health research program in Côte d'Ivoire established Aconda, a local non-profit organization in Abidjan. In June 2004, Aconda launched a five-year program in partnership with the Institute of Public Health, Epidemiology and Development (ISPED, Bordeaux, France) to scale up access to HIV care and treatment. This program was funded by the United States President's Emergency Plan for AIDS Relief (PEPFAR), through the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF, Washington DC, USA).
Here we present data on adults who started ART between June 2004 and November 2006 in the Centre de Prise en Charge et de Formation (CePReF) clinic, Aconda's largest HIV outpatient clinic. The CePReF clinic is located in Yopougon, a low-income suburb of Abidjan, the economic capital of Côte d'Ivoire.
Standardized follow-up procedures
Follow-up procedures for patients on ART in the Aconda Program were described elsewhere [3, 10]. Clinical, immunologic and biochemical evaluations (serum creatinine and transaminases) were completed before ART initiation. Patients initiated ART according to 2003 WHO criteria: clinical stage 4, CD4 count <200/mm3, or clinical stage 3 and CD4 count 200-350/mm3. The Aconda program provided antiretroviral (ARV) drugs to patients monthly. CD4 counts, blood cell counts, serum creatinine and transaminases were measured every six months. Plasma HIV viral load and lactate measurements were not routinely available. Patients paid a fixed rate of US$2 per month for ARV drugs and biological tests, which were made available for their entire immediate family. Support groups were organized to encourage patients to adhere to therapy and a community-based team systematically called or made home visits to patients who did not show up to receive the month's ARV drugs. Cotrimoxazole prophylaxis was prescribed for all patients with CD4 <500/mm3 [11]. Isoniazid (INH) prophylaxis was not included in the national treatment protocol and therefore was not prescribed.
The Aconda Programme used WHO-recommended first-line ARV drugs. When patients were HIV-1-infected, first-line ART consisted of two nucleoside reverse transcriptase inhibitors (NRTI) and one non-nucleoside reverse transcriptase inhibitor (NNRTI). When patients were HIV-2-infected or had dual HIV-1 and HIV-2 infections, first-line consisted of two NRTIs and one protease inhibitor (PI). Stavudine (d4T) dosages (30 mg or 40 mg twice a day) were adapted for patient weight (< or > 60 kg) in accordance with WHO recommendations at the date of the study [12]. Women of child-bearing age who initiated regimens containing efavirenz (EFV) were prescribed hormonal contraception. Antituberculosis drugs were prescribed providing standardized criteria for active tuberculosis, as defined at the national level. The standard antituberculosis treatment consists of an intensive phase of two months daily rifampicin, isoniazid, pyrazinamide and ethambutol, followed by a continuation phase of six months daily isoniazid and ethambutol. Patients on nevirapine who started with a rifampicin-based treatment were switched to efavirenz.
Patient contact with the study centre, drug deliveries and biological test results were recorded anonymously in an electronic database [3]. Before modifying a patient's ART regimen, physicians filled out a standardized form in which they noted the reason for modification. When intolerance was the reason for substituting drugs, adverse effects were scored according to the AIDS Clinical Trial Group's (ACTG) grading table [13]. ARV drugs were substituted according to standardized procedures. Single drug substitutions were recommended whenever possible, except when the cause for regimen modification was ART failure. ART failure was defined based on the WHO criteria for CD4 cell failure (fall of CD4 count to pre-therapy baseline; or 50% fall from the on-treatment peak value; or persistent CD4 levels below 100 cells/mm) and/or the WHO criteria for clinical failure (new/recurrent WHO stage 4 condition) after 12 months of treatment (16). Patients who failed a NNRTI-based first-line regimen with zidovudine or stavudine and lamivudine were given abacavir-didanosine-lopinavir/ritonavir as second-line regimen.
The Aconda computerized data management system has been previously described, and has been approved by the National Ethics Committee of Côte d'Ivoire (3).
Statistical analysis
The primary outcome was first-time ART modification after treatment initiation. ART modifications included changing treatment to alternative first-line regimens (drug substitution for any reason but failure) or second-line regimens (switch for failure). Baseline was defined as the date of ART initiation. Data were censored on November 30 2006 for patients who were alive and actively in care, on the date of death for patients who died before November 30 2006, and on the date of last contact with the care centre for patients who were not known to be dead (transferred out or lost to follow-up) and whose last contact was before November 30 2006. Patients were defined to be lost to follow-up when their last contact with the study team was >3 months on November 30 2006 and if they were not known to be dead or to have transferred out.
Rates of treatment modifications were calculated per 100 patient-years (PY) with 95% confidence intervals. We used the Kaplan-Meier method to estimate the probability of treatment modifications over time, both overall, by drug and by regimen. We used univariate and multivariate Cox proportional hazard regression models for first events to analyze the association between treatment modification and the following characteristics: (i) sex, age, WHO clinical stage, haemoglobin, creatinine or transaminase level, body mass index (BMI) and CD4 count and ART regimen at ART initiation; and (ii) history of tuberculosis before ART initiation, prevalence of tuberculosis at ART initiation and incidence of tuberculosis during follow-up after ART initiation. Sex, age, haemoglobin, CD4 count, BMI and tuberculosis were a priori decided to be included in multivariate analysis regardless of their association with the outcome in univariate analysis. The variables were checked for multicollinearity by examining Tolerance and the Variance Inflation Factor. Analyses were run using SAS software, version 9.1.
Role of the funding source
The sponsors of the treatment program (PEPFAR, EGPAF and Côte d'Ivoire Ministry of Public Health) had no role in the study design, data collection, data analysis, interpretations of data or writing of the report.
Results
Patients
Between June 1 2004 and November 30 2006, 2012 ART-naïve HIV-infected adults (73% women) initiated ART in the CePReF clinic, including 1887 (93.8%) HIV-1 infected patients, 77 (3.8%) HIV-2 infected patients, 36 (1.8%) HIV-1+2 infected patients and 12 (0.6%) patients with no HIV subtyping available. At baseline, median pre-ART CD4 count was 125/mm3, 208 patients (10%) had a self-reported history of active tuberculosis and 182 (9%) had active disease with tuberculosis. The other main characteristics are detailed in Table 1.
Table 1. Initial and follow-up characteristics in the 2012 participants.
| Characteristics at ART initiation | ||
| Women, number (%) | 1472 | (73%) |
| Pregnant women, number (% among women) | 48 | (3%) |
| Age in years, median (IQR) | 36 | (30-42) |
| Body mass index (BMI) in kg/m2, median (IQR) | 19.5 | (17.4-21.6) |
| BMI < 18.5/kg/m2, number (%) | 1213 | (62%) |
| WHO clinical stage 3 or 4, number (%) | 1622 | (82%) |
| History of tuberculosis, number (%) | 208 | (10%) |
| Time since last antiTB treatment in months†, median (IQR) | 18.1 | (9.0-41.8) |
| Active tuberculosis, number (%) | 182 | (9%) |
| CD4 count/mm3, median (IQR) | 125 | (48-211) |
| Haemoglobin level in g/L, median (IQR) | 94 | (82-106) |
| Serum alanine aminotransferase > 50 UI/L, number (%) | 108 | (13%) |
| Creatinine clearance < 80 ml/mn, number (%) †† | 98 | (12%) |
| Initial ART regimen*, number (%) | ||
| 2 NRTIs + 1 NNRTI** | 1902 | (94%) |
| d4T/3TC/NVP, number (%) | 639 | (32%) |
| ZDV/3TC/EFV, number (%) | 759 | (38%) |
| d4T/3TC/EFV, number (%) | 502 | (25%) |
| 2 NRTIs + 1 PI | 109 | (5.4%) |
| 3 NRTIs | 1 | (0.1%) |
| Cotrimoxazole prophylaxis, number (%) | ||
| Started prior to ART initiation | 1858 | (92%) |
| Started after ART initiation | 124 | (6%) |
| Follow-up after ART initiation | ||
| Follow-up time | ||
| Cumulative, person-years | 2659 | |
| Median in months (IQR) | 16.9 | (9.2-22.8) |
| Characteristics at study termination | ||
| Status, number (%) | ||
| Deceased | 205 | (10%) |
| Lost to follow-up | 261 | (13%) |
| Transferred | 122 | (6%) |
Time between last tuberculosis treatment initiation and ART initiation.
Among the 98 (12%) patients with a creatinine clearance < 80 ml/mn, only four had a clearance < 30 ml/mn, 11 had a clearance between 30 and 50 ml/mn and 83 had a clearance between 50 and 80
NRTIs: 3TC (n=2009), ZDV (n=841), d4T (n=1170), ddI (n=3), ABC (n=2); PIs: IDV (n=79), IDV/r (n=23), NFV (n=5), LPV/r (n=2).
Two patients who started with two NRTIs plus one NNRTI received ABC/ddI/EFV (n=1), and ZDV/3TC/NVP (n=1)
IQR: interquartile range; ART: antiretroviral therapy; NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor, WHO: World Health Organisation.
Lost to follow up: patients who were not known to be dead and for whom the time since last contact with the study team was >3 months on 30 November 2006.
Missing values: Age (n=2), BMI (n=54), CD4 count (n=43), WHO clinical stage (n=17), haemoglobin (n=44), alanine aminotransferase (n=1168), creatinine (n=1170). All other variables: n=0.
Of the 2012 patients evaluated, 1902 (94.5%) initiated ART with two NRTIs plus one NNRTI. Of these, 639 (34%) were given a fixed-dose combination of stavudine-lamivudine-nevirapine (Triomune®, Cipla Ldt, India,), 759 (40%) were given a fixed-dose combination of zidovudine-lamivudine (Duovir®, Cipla Ldt, India), with efavirenz (Aurobindo Pharma, India), 502 (26%) were given a combination of stavudine-lamivudine (Lamivir®, Cipla Ldt, India) with efavirenz (Aurobindo Pharma, India), one was given fixed-dose combination of zidovudine-lamivudine (Duovir®, Cipla ldt, India) with nevirapine (Aurobindo Pharma, India), and one was given abacavir (Aurobindo Pharma, India), plus didanosine (Aurobindo Pharma, India), plus efavirenz (Aurobindo Pharma, India). These drugs were delivered under the PEPFAR program or the Global Fund to Fight AIDS, Tuberculosis and Malaria. Overall, 1170 (58%) patients initiated ART with a d4T-containing regimen.
Follow-up
Patients were followed-up for a median of 16.9 months (IQR 9.2-22.8) after ART initiation. By the end of the study, 1424 (71%) patients were alive and actively being followed up, 205 (10%) were known to be dead, 122 (6%) had transferred out and 261 (13%) were lost to follow-up. Median time to death was 2.5 months (IQR 0.9-6.1) and median time to loss to follow-up was 8.2 months (IQR 4.1-11.3).
Treatment modifications
Overall, 483 (24%) patients had a treatment change, at a rate of 20.7/100 patient-years (PY) (95% CI 18.9-22.7) for the first change. Table 2 shows the rates and causes of treatment modifications by initial ART regimen for the three most commonly prescribed regimens. The highest rate of first-time treatment modification was found among patients initiating treatment with a fixed-dose combination of stavudine-lamivudine-nevirapine. Rates of treatment modification were close among patients initiating treatment with stavudine-lamivudine-efavirenz and much lower among patients initiating treatment with zidovudine-lamivudine-efavirenz.
Table 2.
Rates and causes of first treatment modification, by initial ART regimen, for the three most commonly prescribed regimens.
| First-line regimen | ||||
|---|---|---|---|---|
| d4T/3TC/NVP | ZDV/3TC/EFV | d4T/3TC/EFV | ||
| TAR=506 PY | TAR=1089 PY | TAR=625 PY | ||
|
|
||||
| Rate of modification | ||||
| Rate, per 100 PY | 34.0 (28.5-38.7) | 10.5 (8.6-12.4) | 27.2 (23.1-31.3) | |
| Time (months) to first | ||||
| modification, median (IQR) | 4.8 (1.6-8.1) | 6.4 (8.6-12.4) | 12.8 (8.1-16.8) | |
| Cause of modification | ||||
| Intolerance | 80% | 55% | 75 % | |
| Tuberculosis * | 18% | - | 1 % | |
| Pregnancy | - | 36% | 13% | |
| Treatment failure | 1% | 4% | 5% | |
| Drug stock-out | 1% | 5% | 6% | |
TAR: time at risk = cumulative time between the start of the regimen and the first treatment modification (or between the start of the regimen and the end of the study for patients who didn't undergo treatment modification during the study period).
PY: Patient-Year, CI: Confidence Interval, IQR: interquartile range, d4T: stavudine; 3TC: lamivudine; NVP: nevirapine; EFV: efavirenz; ZDV: zidovudine.
episodes of active tuberculosis leading to start a rifampicin-based antituberculosis treatment
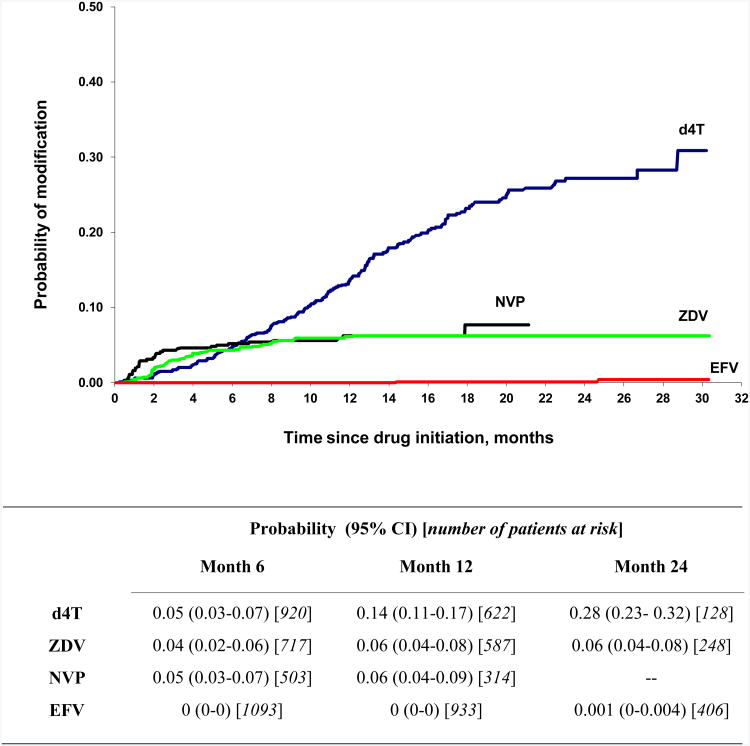
Pregnancy-related substitution of nevirapine for efavirenz accounted for 12.2% of overall treatment modifications, 19.6% of modifications among patients taking efavirenz, and 23.5% of modifications among women taking efavirenz. The rate of treatment modification due to pregnancy in the overall population was 4.5/100 PY (95% CI 3.7-5.3). Switches from nevirapine to efavirenz related to the initiation of a rifampicin-based antituberculosis treatment accounted for 6% of overall treatment modifications and 18% of treatment modifications among patients on nevirapine. The rate of ART modification due to the initiation of a rifampicin-based antituberculosis treatment in the overall population was 2.5/100 PY (95% CI 1.9-3.1). Intolerance was the main cause of treatment modification, with an incidence of 12.4/100 PY (95% CI 11-13.8) in the overall population. Rates of modification for each drug are shown in Table 3. The rates of intolerance-related drug substitutions were very low for efavirenz (0.1/100 PY), low for zidovudine (3.9/100 PY), intermediate for nevirapine (6.3/100 PY) and very high for stavudine (17.9/100 PY). As shown in Table 3 and Figure 1, most intolerance-related drug substitutions occurred during the first few months of treatment, with the exception of stavudine substitutions, which increased steadily over the entire study period.
Table 3. Incidence of intolerance-related drug substitution, by drug, for the four most commonly prescribed drugs.
| d4T | ZDV | NVP | EFV | |
|---|---|---|---|---|
| TAR=1100 PY | TAR=1221 PY | TAR=574 PY | TAR=1940 PY | |
|
|
||||
| Rate, per 100 PY (95% CI) | 17.9 (15.4-20.4) | 3.9 (2.8-8.3) | 6.3 (4.3-8.3) | 0.1 (0-0.2) |
| Time (in months) to first modification, median (IQR) | 10 (6-13.2) | 3.1 (1.9-6.4) | 1.5 (0.9-3.2) | 19.6 (17-22.1) |
TAR: time at risk = cumulative time between the start and the substitution of the drug (or between the start of the drug and the end of the study for patients who did not substitute the drug during the study period)
PY: Patient-years, CI: Confidence Interval, IQR: interquartile range, d4T: stavudine; NVP: nevirapine; EFV: efavirenz; ZDV: zidovudine.
Figure 1. Cumulative probability of intolerance-related drug substitution over time, by drug.
CI: Confidence Interval
d4T: stavudine; NVP: nevirapine; EFV: efavirenz; ZDV: zidovudine.
As shown in Table 1, drug stockouts were a non-negligible cause of treatment modification. Patients were sometimes forced to change treatment in order to avoid treatment interruptions. Although stockouts accounted for only 1% of stavudine-lamivudine-nevirapine modifications, they represented 5-6% of modifications for other regimens.
The factors associated with intolerance-related treatment modifications are shown in Table 4. Neither the tolerance values (all >0.2) nor the Variance Inflation Factor statistics (all <4) indicated a problem of multicollinearity for any of the variables in the multivariate analysis. In multivariate analysis, d4T substitutions due to intolerance (mainly, peripheral neuropathy) were significantly associated with age. We did not find a significant association between d4T substitution and previous or ongoing anti-tuberculosis treatment. Substitution of zidovudine was strongly associated with baseline haemoglobin and baseline anti-tuberculosis treatment. We did not find significant associations between nevirapine substitution and any of the baseline or follow-up patient characteristics.
Table 4. Factors associated with intolerance-related treatment modifications in patients whose first-line ART regimen contained d4T, NVP or ZDV: a multivariate analysis.
| d4T | NVP | ZDV | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Characteristics at ART initiation | |||||||||
| Sex women (ref: male) | 0.82 | 0.56; 1.20 | 0.30 | 0.56 | 0.21; 1.50 | 0.25 | 1.10 | 0.58; 2.08 | 0.77 |
| Age (for one year more) | 1.04 | 1.02; 1.06 | <.0001 | 1.00 | 0.97; 1.05 | 0.79 | 1.02 | 0.98; 1.05 | 0.24 |
| CD4 count < 150/mm3 (ref >150/mm3) | 1.43 | 1.05; 1.96 | 0.02 | 0.97 | 0.48; 1.92 | 0.93 | 0.98 | 0.54; 1.77 | 0.95 |
| Tuberculosis (TB) * | 0.97 | 0.97 | 0.04 | ||||||
| History of TB (ref = no TB) | 1.02 | 0.64; 1.63 | 0.95 | 0.22; 4.06 | 2.13 | 0.99; 4.58 | |||
| Active TB (ref = no TB) | 1.07 | 0.62; 1.83 | 0.77 | 0.08; 6.94 | 2.36 | 1.06; 5.24 | |||
| Haemoglobin level (for 10 g/L higher) | 1.09 | 0.99 ; 1.20 | 0.08 | 0.96 | 0.77; 1.19 | 0.68 | 0.76 | 0.63; 0.91 | 0.002 |
| Events during follow-up ** | |||||||||
| Incident active tuberculosis (ref = no) | 1.80 | 0.86; 3.76 | 0.12 | 2.29 | 0.45; 11.48 | 0.31 | 2.55 | 0.34; 18.87 | 0.36 |
CI: Confidence Interval, HR: hazard ratio; d4T: stavudine; NVP: nevirapine; EFV: efavirenz; ZDV: zidovudine; ref: reference
TB at ART-initiation was categorised into three categories (No TB; A history of TB; Active TB); the p-values shown on the TB row are the global p-values;
follow-up TB was assessed at each visit and was included in the analysis as a time updated variable. For each episode of active TB, the date of the episode was the date of the first symptoms.
Discussion
We report here on the incidence, risk factors and causes of first-line ART regimen modifications in 2012 HIV-infected adults who were followed for a median of 17 months after ART initiation during the first two years of an ART roll-out program in Côte d'Ivoire.
As expected, regimen switches due to failure were rare during the early phase of ART and treatment modifications were mainly due to intolerance. However, we also found that a third of regimen modifications were related not to intolerance or treatment failure, but rather to pregnancies and tuberculosis, which frequently led to efavirenz and nevirapine substitutions, respectively.
NNRTIs are essential to first-line ART regimens in sub-Saharan Africa. When nevirapine and efavirenz are available, physicians must decide which drug to prescribe, based on the cost of the drug, the risk of intolerance to the drug, the existence of a fixed-combination pill and the patient's pre-ART characteristics. In settings with a substantial prevalence of tuberculosis and proportion of HIV-infected women of childbearing age, both nevirapine and efavirenz have a high likelihood of being substituted for the other for reasons other than intolerance. On the one hand, although ART does reduce the incidence of tuberculosis, it remains the leading severe opportunistic infection during the first years on ART [14]. Some studies have explored the possibility of using nevirapine and rifampicin concomitantly [15], but both WHO guidelines and government directives in countries like Côte d'Ivoire still recommend the substitution of efavirenz for nevirapine during rifampicin therapy [16]. On the other hand, efavirenz has a potential embryo/foetotoxicity. Prescribing contraceptives in women taking efavirenz is only part of the solution, as various studies have shown that the rate of pregnancy in women taking contraceptives remains significant [17, 18]. In additions, pregnancy rates in women taking ART will likely increase in the future, as ART helps CD4 counts rise [19].
The clinical consequences of switching from one NNRTI to another are likely to be limited at the individual level and under good conditions of care, but they still deserve to be studied in settings where resources for managing intolerance and non-adherence are limited. Furthermore, high rates of nevirapine and efavirenz crossover may hinder drug forecasting at the program level. In sub-Saharan Africa, drug forecasting and stable drug management systems are crucial to ensuring the success of HIV treatment programs [20]; reductions in rates of drug switches could contribute to the success of ART delivery programs.
So far, only a handful of articles have reported on the rates and causes of first-time ART regimen modifications due to intolerance in sub-Saharan Africa [8, 17, 21]. These studies all focused on treatment modification, and not drug toxicity. Though the rate of intolerance-related treatment modification does likely reflect the rate of severe toxicity events, it only acts as an imperfect proxy. In our setting, fatal toxicity may remain undiagnosed, with patients dying or withdrawing from care before switching drugs. Furthermore, limited access to care, and second-line ART, as well as infrequent diagnoses of side effects, curtail the patient's ability to switch drugs [9], perhaps explaining the relatively low rates of treatment modification we report, compared to those reported in developed countries [22-25]. Finally, in our study 13% of patients were lost to follow-up and were censored at the date of last contact with the centre. This censoring could be informative, thus leading us to misestimate the rate of severe drug toxicity and/or the rate of treatment failure.
Within the limitations stated above, the rates of toxicity and times to regimen modification reported here are consistent with those previously reported in sub-Saharan African countries [8, 17, 21]. As expected, stavudine was associated with the highest rate of and longest time to regimen modification. In our context, the diagnosis of lactic acidosis is rarely made [26]. The most common causes of stavudine discontinuation in routine care are lipodystrophy and peripheral neuropathy. Although over-diagnosis of these adverse events may have occurred in our study, some patients who were intolerant to stavudine may not have substituted the drug because they did not have easy access to the health care facility, or second-line drugs were not available. As expected, intolerance-related drug discontinuations were much lower for efavirenz and zidovudine, compared with nevirapine and stavudine, respectively.
Low pre-ART CD4 cell count and older age were significantly associated with stavudine-containing regimen modifications and pre-ART anaemia was associated with zidovudine-containing regimen modifications [27, 28]. The design of the study, sample size and limited number of variables, however, do limit our interpretation of the factors associated with toxicity-related regimen modifications. Some key variables such as prevalence of hepatitis B virus co-infection are missing and may have acted as confounding factors [29].
In conclusion, we have found evidence that rates of both pregnancy-related efavirenz discontinuations and tuberculosis-related nevirapine discontinuations are high in this population of West African adults, in which the proportion of women is large and for which both drugs are equally available. In addition, we found that a remarkably low rate of efavirenz discontinuations was associated with drug intolerance. Rates of ART regimen modifications in patients taking efavirenz and any NRTI except for stavudine would be strikingly low if efavirenz were shown to be safe for pregnant women. If new data are not reported soon supporting the use of efavirenz during pregnancy or the use of nevirapine during tuberculosis treatment, the dilemma between these two NNRTIs will persist. Instead, other, newer drugs that do not require interruptions during tuberculosis or pregnancy will have to be made more readily available as first-line drugs in sub-Saharan Africa.
Acknowledgments
Participants in the study were treated in a program funded by the Côte d'Ivoire Ministry of Public Health and the United States President's Emergency Plan for AIDS Relief (PEPFAR), through the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF, Washington DC, USA); The study was funded by the French National Agency for research on AIDS and viral hepatitis (ANRS, France, grant 1203).
List of the Centre de Prise en Charge et de Formation (CePReF) health workers: Pierre Aka, Amani Anzian, Nicole Dakoury-Dogbo, Mamadou Diarrassouba, Lambert Konan, Sidonie Dohoun-Kosseassé, Eric Konan-N'Dri, Joachim Gnokoro, Jeanot Goli, Patrick Gouessé, Marie-Cécile Kassi, Agnès Kati, Eric Komena, Lucie Konan, Michel Konan, Georgette Labibi, Eugène Messou, Yves Mobio, Denis Niamien, Marie-Pascale Nogbou, Abou Sorho, Adidiata Soro, Amah Tchehy, Landry Yepié, Agnès Yoman, Zamblé-Bi.
Footnotes
Contributors: X Anglaret, T N'Dri-Yoman, C Seyler, A Tanoh sought funding for the study. E Komena, E Messou and C Seyler were responsible for overall study coordination. J Duvignac and E Konan-N'Dri were the study statisticians, X Anglaret, E Messou and C Seyler drafted the manuscript, which all authors subsequently reviewed, edited and approved.
Conflict of interest: None
References
- 1.UNAIDS, WHO. AIDS epidemic update: Sub-Saharan Africa regional summary. 2007 http://data.unaids.org/pub/Report/2008/jc1526_epibriefs_ssafrica_en.pdf.
- 2.WHO, UNAIDS, UNICEF. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress Report, 2008. http://www.who.int/hiv/pub/towards_universal_access_report_2008.pdf.
- 3.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d'Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 5.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 6.Fairall LR, Bachmann MO, Louwagie GM, van Vuuren C, Chikobvu P, Steyn D, et al. Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 7.Wester CW, Kim S, Bussmann H, Avalos A, Ndwapi N, Peter TF, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40:336–343. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 8.Boulle A, Orrel C, Kaplan R, Van Cutsem G, McNally M, Hilderbrand K, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12:753–760. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 9.Forna F, Liechty CA, Solberg P, Asiimwe F, Were W, Mermin J, et al. Clinical toxicity of highly active antiretroviral therapy in a home-based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr. 2007;44:456–462. doi: 10.1097/QAI.0b013e318033ffa1. [DOI] [PubMed] [Google Scholar]
- 10.Seyler C, Adje-Toure C, Messou E, Dakoury-Dogbo N, Rouet F, Gabillard D, et al. Impact of genotypic drug resistance mutations on clinical and immunological outcomes in HIV-infected adults on HAART in West Africa. AIDS. 2007;21:1157–1164. doi: 10.1097/QAD.0b013e3281c615da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anglaret X, Messou E, Ouassa T, Toure S, Dakoury-Dogbo N, Combe P, et al. Pattern of bacterial diseases in a cohort of HIV-1 infected adults receiving cotrimoxazole prophylaxis in Abidjan, Cote d'Ivoire. AIDS. 2003;17:575–584. doi: 10.1097/00002030-200303070-00013. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Scalling up antiretroviral therapy in ressource-limited settings: Treatment guidelines for a public health approach. 2003 revision. http://www.who.int/hiv/pub/prev_care/en/arvrevision2003en.pdf.
- 13.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. MMWR Recomm Rep. 2002;51:1–55. [PubMed] [Google Scholar]
- 14.Moh R, Danel C, Messou E, Ouassa T, Gabillard D, Anzian A, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21:2483–2491. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- 15.Manosuthi W, Ruxrungtham K, Likanonsakul S, Prasithsirikul W, Inthong Y, Phoorisri T, et al. Nevirapine levels after discontinuation of rifampicin therapy and 60-week efficacy of nevirapine-based antiretroviral therapy in HIV-infected patients with tuberculosis. Clin Infect Dis. 2007;44:141–144. doi: 10.1086/510078. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Antiretroviral therapy for HIV infection in adults and adolescents in ressource-limited settings: towards universal access. Recommendations for a public health approach. 2006 revisions. http://www.who.int/hiv/pub/guidelines/WHO%20Adult%20ART%20Guidelines.pdf.
- 17.Danel C, Moh R, Anzian A, Abo Y, Chenal H, Guehi C, et al. Tolerance and acceptability of an efavirenz-based regimen in 740 adults (predominantly women) in West Africa. J Acquir Immune Defic Syndr. 2006;42:29–35. doi: 10.1097/01.qai.0000219777.04927.50. [DOI] [PubMed] [Google Scholar]
- 18.Bussmann H, Wester CW, Wester CN, Lekoko B, Okezie O, Thomas AM, et al. Pregnancy rates and birth outcomes among women on efavirenz-containing highly active antiretroviral therapy in Botswana. J Acquir Immune Defic Syndr. 2007;45:269–273. doi: 10.1097/QAI.0b013e318050d683. [DOI] [PubMed] [Google Scholar]
- 19.Loko MA, Toure S, Dakoury-Dogbo N, Gabillard D, Leroy V, Anglaret X. Decreasing incidence of pregnancy by decreasing CD4 cell count in HIV-infected women in Cote d'Ivoire: a 7-year cohort study. AIDS. 2005;19:443–445. doi: 10.1097/01.aids.0000161776.30815.44. [DOI] [PubMed] [Google Scholar]
- 20.Harries AD, Schouten EJ, Makombe SD, Libamba E, Neufville HN, Some E, et al. Ensuring uninterrupted supplies of antiretroviral drugs in resource-poor settings: an example from Malawi. Bull World Health Organ. 2007;85:152–155. doi: 10.2471/BLT.06.032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tonwe-Gold B, Ekouevi DK, Viho I, Amani-Bosse C, Toure S, Coffie PA, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLoS Med. 2007;4:e257. doi: 10.1371/journal.pmed.0040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.d'Arminio Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. AIDS. 2000;14:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 23.Mocroft A, Youle M, Moore A, Sabin CA, Madge S, Lepri AC, et al. Reasons for modification and discontinuation of antiretrovirals: results from a single treatment centre. AIDS. 2001;15:185–194. doi: 10.1097/00002030-200101260-00007. [DOI] [PubMed] [Google Scholar]
- 24.Kirk O, Gerstoft J, Pedersen C, Nielsen H, Obel N, Katzenstein TL, et al. Low body weight and type of protease inhibitor predict discontinuation and treatment-limiting adverse drug reactions among HIV-infected patients starting a protease inhibitor regimen: consistent results from a randomized trial and an observational cohort. HIV Med. 2001;2:43–51. doi: 10.1046/j.1468-1293.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith CJ, Sabin CA, Lampe FC, Shah S, Tyrer M, Youle MS, et al. The relationship between CD4 cell count nadirs and the toxicity profiles of antiretroviral regimens. Antivir Ther. 2005;10:459–467. [PubMed] [Google Scholar]
- 26.Mutimura E, Stewart A, Rheeder P, Crowther NJ. Metabolic function and the prevalence of lipodystrophy in a population of HIV-infected African subjects receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46:451–455. doi: 10.1097/qai.0b013e318158c0a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenstein KA, Armon C, Baron A, Moorman AC, Wood KC, Holmberg SD. Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV outpatient study cohort. Clin Infect Dis. 2005;40:148–157. doi: 10.1086/426076. [DOI] [PubMed] [Google Scholar]
- 28.Moh R, Danel C, Sorho S, Sauvageot D, Anzian A, Minga A, et al. Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Cote d'Ivoire. Antivir Ther. 2005;10:615–624. doi: 10.1177/135965350501000510. [DOI] [PubMed] [Google Scholar]
- 29.Dieterich DT, Robinson PA, Love J, Stern JO. Drug-induced liver injury associated with the use of nonnucleoside reverse-transcriptase inhibitors. Clin Infect Dis. 2004;38(Suppl 2):S80–89. doi: 10.1086/381450. [DOI] [PubMed] [Google Scholar]