Abstract
The concept of the heart as a terminally differentiated organ incapable of replacing damaged myocytes has been at the center of cardiovascular research and therapeutic development for the past 50 years. The progressive decline in myocyte number as a function of age and the formation of scarred tissue after myocardial infarction have been interpreted as irrefutable proofs of the postmitotic characteristic of the heart. However, emerging evidence supports a more dynamic view of the heart in which cell death and renewal are vital components of the remodeling process that governs cardiac homeostasis, aging, and disease. The identification of dividing myocytes in the adult and senescent heart raises the important question concerning the origin of these newly formed cells. In vitro and in vivo findings strongly suggest that replicating myocytes derive from lineage determination of resident primitive cells, supporting the notion that cardiomyogenesis is controlled by activation and differentiation of a stem cell compartment. It is the current view that the myocardium is an organ permissive of tissue regeneration mediated by exogenous and endogenous progenitor cells.
A fundamental issue pertaining to the ability of the heart to sustain cardiac diseases of ischemic and nonischemic origin is whether myocardial regeneration occurs in the adult organ or whether this growth adaptation is restricted to prenatal life, severely limiting the response of the myocardium to pathologic stresses.1 The concept of the heart as a terminally differentiated organ incapable of replacing damaged myocytes has been at the center of cardiovascular research for the past 50 years.2–6 The accepted view has been that the postnatal, adult, and aging heart reacts to an increase in workload by hypertrophy of existing myocytes only. Hypertrophied cardiomyocytes express the senescence-associated proteins p16INK4a and p537–11 and are prone to undergo apoptosis and necrosis, possibly because of the high intracellular content of reactive oxygen species. Cell death is restricted to p16INK4a-positive myocytes, but the process is inefficient, resulting in the progressive accumulation of poorly functional myocytes9; they are characterized by changes in the expression of contractile protein isoforms and by profound alterations in intracellular calcium cycling and electrical properties.12–14 These defects, together with myocyte loss and the development of foci of myocardial scarring, inevitably contribute in the long term to the onset of ventricular dysfunction and its progression to overt failure.
REGENERATIVE CAPACITY OF ADULT ORGANS
The ability of stem cells to continuously replenish the compartment of undifferentiated and lineage-committed cells is a typical property of higher organisms with mitotic soma. In these cases, tissues are capable of renewal and repair, and the extent of regeneration positively correlates with animal lifespan.15,16 The reduced longevity of lower organisms, including Caenorhabditis elegans and Drosophila, is linked to the lack of regenerative potential of their postmitotic tissues in adulthood.15 Dying cells cannot be replaced, leading to a rapid decline in organ function. Conversely, cell turnover by proliferation and commitment of resident progenitor cells is active in mammals, and old injured cells lost as a result of normal wear and tear may be restored by new, better-functioning cells. However, regeneration is hampered in the presence of damage, which creates a barrier to restitutio ad integrum and promotes a repair process leading to the formation of a scar.1,17 The scar does not possess the biochemical, physical, and functional properties of the intact tissue, negatively affecting the remodeling of the diseased heart, a critical determinant of cardiac performance.17,18
Despite the presence of resident adult stem cells, the repair mechanism triggered by ischemic injury results in collagen accumulation and scarring. This phenomenon is not restricted to the heart but occurs in all the organs, whether their parenchymal cells are highly proliferating, slowly cycling, or terminally differentiated. This intrinsic limitation was interpreted as the inability of the adult myocardium to generate de novo cardiomyocytes.16,17 However, the outcome of infarction is essentially identical throughout the organism. Occlusion of a major conductive artery or a large branch results in loss of tissue in the skin, kidney, intestine, brain, liver, and reproductive organs in a manner identical to the heart.17,19–23 This common outcome is conditioned by 2 crucial factors: (1) stem cells in the infarct die as do all other cells deprived of oxygen supply and (2) resident stem cells activate a local regenerative response but do not migrate from the viable tissue to the damaged area to replace the necrotic tissue (Figure 1).
FIGURE 1.
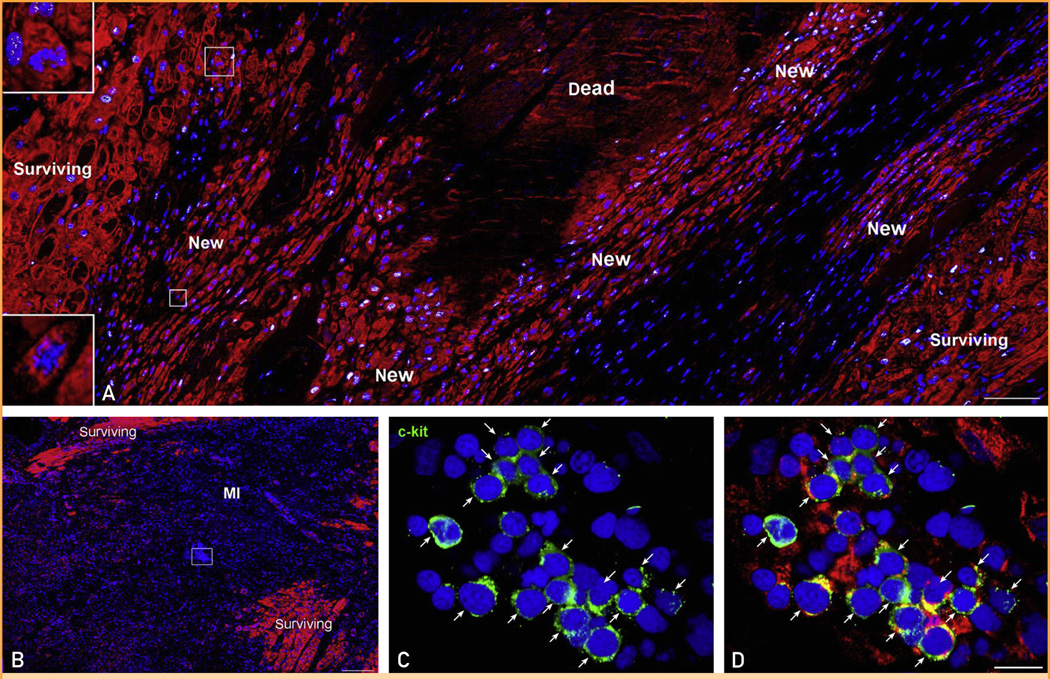
Heart failure and myocardial regeneration. A, Area of regenerating myocardium in the infarct. Proliferating, small, developing myocytes are positive for the cell-cycle marker MCM5 (white). Myocytes are labeled by cardiac myosin (red) and nuclei by DAPI (blue). Two small myocytes in mitosis are shown in the insets (magnification 300×). B, The cluster of cells included in a rectangle in the middle of an acute infarct is shown at higher magnification in C and D. These cells express c-kit (green, arrows) and at times cardiac myosin (red; D), reflecting undifferentiated cardiac stem cells and early committed progenitors, respectively. MI = myocardial infarction. Scale bars: A and B, 100 µm; C and D, 10 µm.
The main factors that dictate the evolution of a lesion into scarring rather than regeneration have not been identified with certainty. Some insights come from embryonic development. Repair in embryos is rapid, efficient, and scar free.17 Skin wounds in the early mammalian embryo heal with restitutio ad integrum, whereas wounds in late gestational fetuses and adult mammals result in scarring. This difference may be related to the intrinsic characteristics of embryonic fibroblasts or to external stimuli. Fibroblasts in embryos are less prone to synthesize and release collagen, attenuating the amount of fibrosis after injury.17,24 The presence of hyaluronic acid and fibronectin in the amniotic fluid may also interfere with scar formation during wound healing. Moreover, the inflammatory response is attenuated; a low number of poorly differentiated inflammatory cells accumulate in the region of damage, and the growth factors present at the site of healing are different from those in the adult organism.17,25–27 Scar-free regeneration of damaged organs is well known in amphibians and zebra fish.17,28 Epimorphic regeneration corresponds to the complete restoration of organs after the development of body plans and cellular differentiation.16,17,29 However, this occurs only in invertebrates and lower vertebrates, including urodeles and fish. Conversely, mammals retain limited capacity for spontaneous regeneration in the adult liver and infant fingertips,16,17,29,30 suggesting that during evolution animals gradually lose their ability to reconstitute damaged organs. Characterization of the processes that drive regeneration in lower vertebrates may provide some information for the implementation of regenerative strategies in humans. Regeneration of the adult heart occurs in newts and zebra fish and has been claimed to involve the reentry of cardiomyocytes into the cell cycle31,32 or dedifferentiation.11
Dedifferentiation refers to a regression of a mature cell in its own lineage; this process is typically coupled with attainment of the proliferative capacity. Dedifferentiation is considered the natural regenerative response to cardiac injury in zebra fish, newts, and planaria. After disassembly of the sarcomeric contractile apparatus, which impedes cytokinesis, differentiated cardiomyocytes replicate to reconstitute up to 20% of the zebra fish ventricle after resection. 11 By cre/lox cell lineage analysis, the regenerating myocardium has been shown to derive primarily from a subpopulation of GATA4-positive cardiomyocytes rather than from nonmyocyte sources, such as stem cells. The poorly organized sarcomeric structure of replicating GATA4-positive myocytes has led to the hypothesis that dedifferentiation constitutes the primary regenerative step.32 However, the failure in the induction of the early myocyte commitment genes Nkx2.5 and Hand2 in the replicating myocytes indicate that the cells of origin underwent limited dedifferentiation or, most likely, that replicating GATA4-positive cardiomyocytes correspond to cells at the late stage of commitment of stem cell differentiation. In the mouse heart, Nkx2.5 expression precedes GATA4 in transit-amplifying cardiomyocytes originated by lineage specification of cardiac stem cells (CSCs) (Figure 2).33
FIGURE 2.
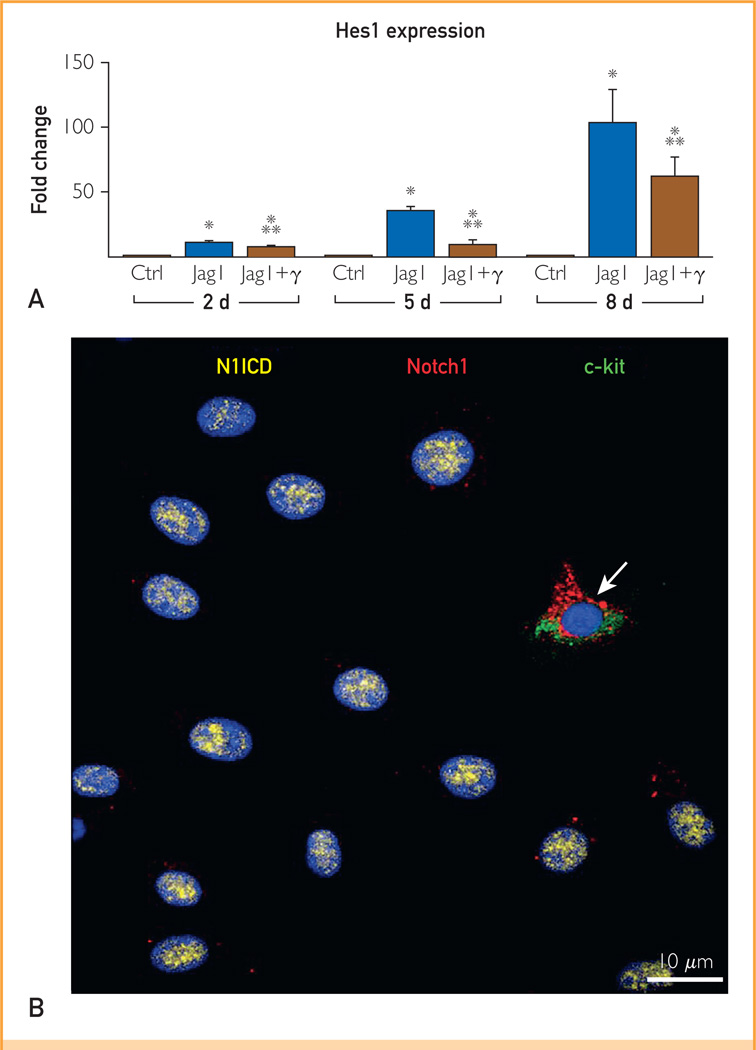
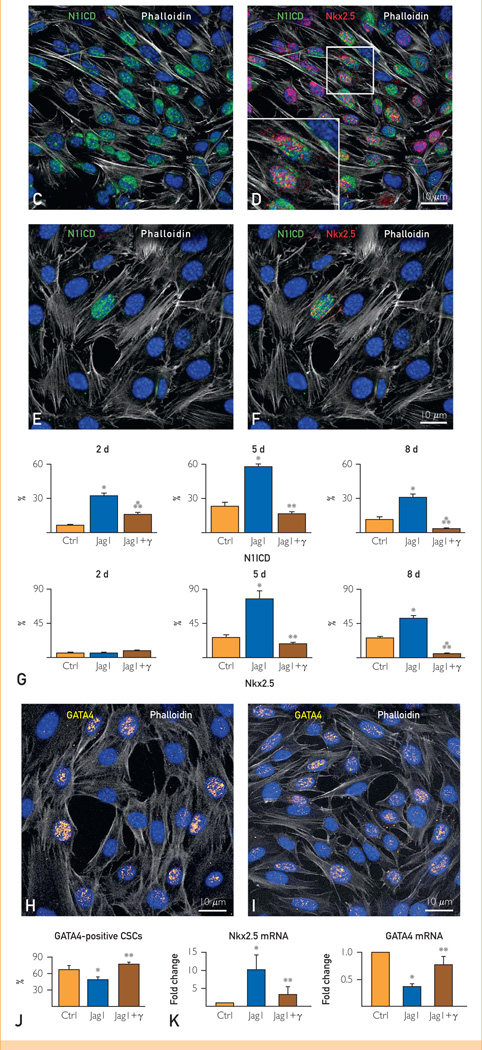
Notch1 up-regulates Nkx2.5 but not GATA4 during early commitment of cardiac stem cells (CSCs). A, Hes1 transcript was measured in CSCs at 2, 5, and 8 days under control conditions (Ctrl) and in the presence of Jagged1 (Jag1) and Jag1 and γ-secretase inhibitor (Jag1 + γ). Hes1 quantity is shown as fold changes vs the corresponding Ctrl. B, After Jag1 stimulation, CSCs are c-kit negative and display nuclear localization of the active fragment of Notch1 (Notch1 intracellular domain [N1ICD], yellow). One CSC continues to express c-kit (green) together with the extracellular domain of the Notch1 receptor (red, arrow). C and D, Jag1-stimulated CSCs express N1ICD (green) together with Nkx2.5 (red). The area included in the square is shown at higher magnification in the inset (magnification: 1000×). E and F, After γ-secretase inhibition, Jag1-stimulated CSCs are negative for N1ICD and Nkx2.5. G, Percentage of CSCs positive for N1ICD and Nkx2.5 at 2, 5, and 8 days. H and I, The expression of GATA4 (yellow) is shown in CSCs stimulated by Jag1 in the (H) absence and (I) presence of γ-secretase inhibitor. J, Percentage of CSCs positive for GATA4 at 5 days. K, Nkx2.5 and GATA4 transcripts were analyzed in CSCs at 5 days. mRNA = messenger RNA. *P<.05 vs Ctrl. **P<.05 vs Jag1. ***Statistically different vs both Ctrl and Jag1. Data are shown as mean ± SD.
A comparable regenerative response has been observed after surgical resection of the apex of the left ventricle in the neonatal mouse heart.34 Again, cardiomyocyte proliferation was considered as the cellular adaptation supporting the repair process. Genetic fate mapping strategies have been used to distinguish the contribution of preexisting myocytes and stem/progenitor cells to regeneration of the zebra fish and mouse heart. This approach, however, has several intrinsic limitations. The specificity of the promoter and its transactivated protein for the population of cells to be studied is an essential prerequisite of lineage tracing. Cardiac stem cells engineered with fluorescent constructs placed under the control of promoters encoding myocyte-specific transcription factors and sarcomeric proteins show transgene expression.35 Although GATA4 and α-myosin heavy chain promoters are generally considered to be active primarily in cardiomyocytes, the presence of the reporter gene in c-kit—positive CSCs reflects a very early stage of cardiogenic lineage commitment of these mother cells, which, after the loss of the stem cell antigen, give rise to a large compartment of transit-amplifying myocytes (Figure 3). Thus, the lack of specificity severely hampers the validity of the findings obtained with fate mapping strategies when promoters encode alleged myocyte-restricted proteins.10,11
FIGURE 3.
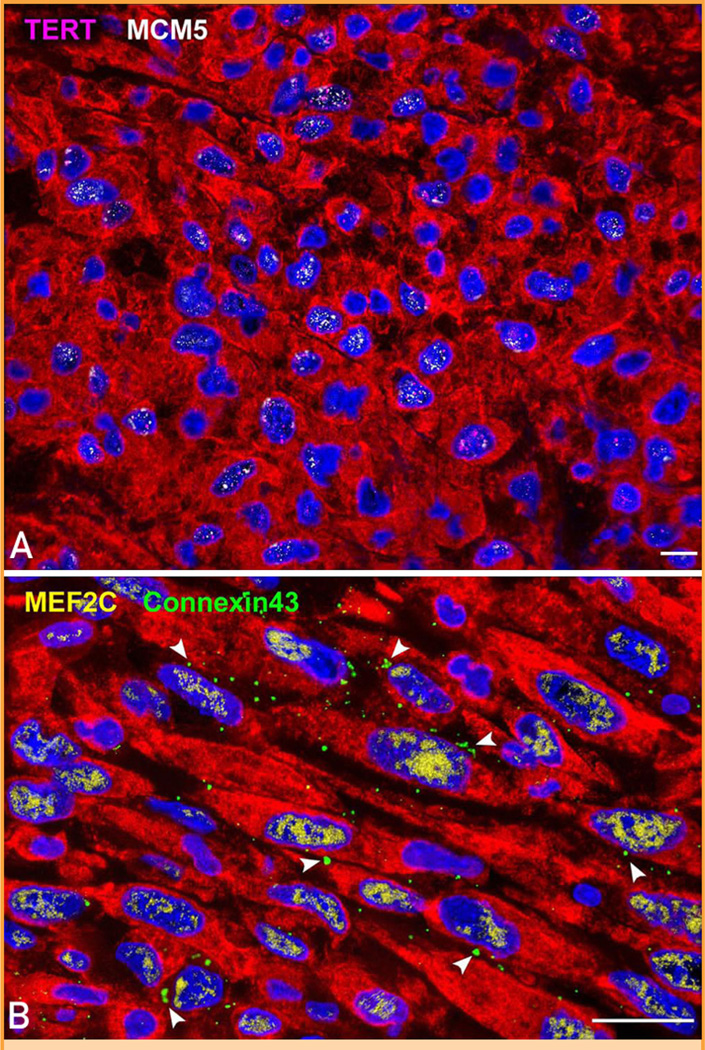
Transit-amplifying myocytes. Small developing myocytes in the infarct are positive for (A) telomerase (magenta) and MCM5 (white) and for (B) MEF2C (yellow) and connexin 43 (green, arrowheads). TERT = telomerase reverse transcriptase. Scale bars, 10 mm.
The essential foundation of meticulously performed lineage tracing protocols consists of expression of the fluorescent label in the pool of cells hierarchically located upstream of the progeny to be tracked; the use of promoters regulating contractile proteins introduces a serious bias into the method. The availability of cre/lox mice carrying fluorescent tags under the control of stem cell antigens will clarify this controversial issue. Data collected in mice with spontaneous inactive mutations of c-kit and mice carrying a deletion of Sca-1 strongly suggest that stem cells contribute significantly to cardiomyogenesis during homeostasis and regulate the magnitude of myocyte regeneration after injury.36–38 Thus, current observations in zebra fish and neonatal, adult, and old mice do not exclude that CSCs participate in myocardial growth.
Evidence of dedifferentiation, ie, reentry of terminally differentiated cardiomyocytes into the cell cycle, is based exclusively on cellular morphology. Cells characterized by an intermediate phenotype between forming and mature myocytes have arbitrarily been considered the product of dedifferentiation.10,11,39 However, the lack of structural markers typical of a given cell type is not by itself indicative of the developmental stage of the cell10; it is impossible to define in vivo whether the cell of interest is undergoing differentiation or whether it is in the process of reverting to an earlier immature state. As discussed previously herein, the possibility of dedifferentiation was raised by observations conducted in vertebrates with exceptional regenerative abilities. This conclusion, however, has recently been challenged. During salamander limb reconstitution, cells from muscle, bone, cartilage, nerve sheath, and connective tissues are believed to dedifferentiate into a pool of proliferating cells known as the regeneration blastema.40–42 But the possibility that preexisting unipotent or multipotent stem cells constitute the pool of proliferating blastema cells43 questions the notion of dedifferentiation and subsequent redifferentiation of a common pool of cells responsible for the repair of the amputated limb.43 Similarly, the hypothesis has been raised that clonally dominant cardiomyocytes with properties similar to those of stem cells direct heart morphogenesis in zebra fish from the early stages of development to adulthood.44
MYOCARDIAL REGENERATION: THE CONTROVERSY
A critical issue of cardiac pathobiology concerns whether myocardial regeneration occurs in the adult organ or whether this growth adaptation is restricted to prenatal life and ceases shortly after birth.1–6,10,11 The concept that the heart is a postmitotic, terminally differentiated organ unable to replace its myocyte compartment has been at the center of cardiovascular research for several decades.1–6,10,11,45 The accepted view is that the myocardium reacts to an increased workload by hypertrophy of the existing myocytes, and when this cellular response is exhausted, ventricular dysfunction supervenes. Based on this assumption, molecular cardiology has focused in the past 30 years on identification of the multiple signaling pathways regulating the activation and depression of genes implicated in the hypertrophic growth of cardiomyocytes during postnatal maturation or after abnormal pathologic states or chronological aging.1,10,11,17,45–50 The possibility that the heart can renew its parenchymal cells has been largely dismissed, and, even today, myocardial repair is viewed with suspicion and trepidation.40,41
The engrained paradigm that promotes a rather uninteresting biological perspective of the developing, old, and diseased heart has been shaken by a variety of studies indicating that myocyte regeneration occurs in humans and animals after infarction,8,51 after prolonged pressure overload,7 and in the decompensated senescent heart.52,53 Although some of these studies were published almost 25 years ago54 and systematically continued to appear in the literature (for reviews, see references 10, 11, 40, and 41), the traditional establishment tends to reject this alternative notion of cardiac biology, defending a territory that was considered unwavering and immovable.
The recognition that myocyte death by apoptosis and necrosis occurs continuously in the adult heart of humans and animals9,17,55,56 emphasizes the necessity for an equivalent degree of myocyte formation to preserve cardiac mass and function. The discovery of resident CSCs and their ability to differentiate into cardiomyocytes, vascular endothelial cells, and smooth muscle cells has provoked a rather negative response in part of the scientific community; CSCs were either ignored or claimed to lack biological significance.5,57,58 Similarly, the documentation that hematopoietic stem cells transdifferentiate, generating cardiomyocytes and coronary vessels after infarction,1,59,60 was immediately rejected, prompting a series of negative studies that challenged the validity of the early observations.61,62 Some comments about the history of the heart as a postmitotic organ may be relevant for understanding the shift in paradigm required for the implementation of the novel field of regenerative cardiology.
THE HEART AS A POSTMITOTIC ORGAN
Numerous studies of the human heart from 1850 to 1911 held the view that myocardial hypertrophy was the consequence of hyperplasia and hypertrophy of existing myocytes.10,46,63–65 However, subsequent reports from 1921 to 1925 questioned the ability of myocytes to proliferate, suggesting that the increase in cardiac muscle mass in the pathologic heart was the result of pure cellular hypertrophy.10,46,66–68 The concept that myocytes cannot divide originated from difficulty in identifying mitotic figures in these cells in the absence of quantitative evaluations of myocyte size and number.68 This conviction gained support from autoradiographic analysis of thymidine incorporation in the hearts of animals during postnatal growth69 and after pressure overload.70–72 DNA synthesis in myocyte nuclei was not detected, prompting the conclusion that the heart survives and exerts its function until the death of the organism with the same cells that are present at birth.73
Accordingly, ventricular myocytes in humans are terminally differentiated cells, and their life-span corresponds to the lifespan of the organism. The number of myocytes attains an adult value a few months after birth,74 and the same myocytes are believed to contract 70 times per minute throughout life. Because a certain fraction of the population reaches 100 years of age or more, an inevitable consequence of this paradigm is that cardiac myocytes are immortal, functionally and structurally.1,10,17,46
In view of this conviction, we discuss myocardial aging and the potential mechanisms involved in acquisition of the cardiac senescent phenotype. In addition, we address the differences in the rate of myocyte turnover claimed by various laboratories and the potential role that resident human CSCs have in organ homeostasis and regeneration as a function of age. This information is critical to define whether the aging myopathy is a stem cell disease, and strategies can be developed to modify the progression of ventricular dysfunction in the old heart.75
MYOCARDIAL AGING IN HUMANS
The aging myopathy typically manifests itself with diastolic dysfunction and preserved ejection fraction (EF).76 More than 50% of patients with heart failure have normal or near-normal EF, and the incidence and prevalence of this condition increases with age.76–80 Although the claim is commonly made that age-associated physiologic changes predispose older adults to development of heart failure with a normal EF, the etiology of diastolic heart failure is unknown. The difficulty in defining myocardial aging and the mechanisms involved further complicates recognition of the cellular processes underlying impaired diastolic relaxation. Morbidity and mortality for chronic heart failure continue to increase and parallel the extension in median life-span of the population,81 pointing to aging as the major risk factor of the human disease. At present, there are 5.1 million patients with chronic heart failure in the United States alone, with an incidence of 670,000 new cases per year,82 and most individuals with chronic heart failure are 65 years or older.
Currently, we have little understanding of the etiology of myocardial aging. Rarely, studies in animals and humans have considered aging as an independent process and time as the major cause of the manifestations of the aging myopathy. Aging has been interpreted as a variable, which cooperates with a variety of diseases, to define the old heart.83–85 Only occasional reports have characterized cardiac aging in humans independent of concomitant pathologic states.9,53,86 Myocardial aging differs in women and men, emphasizing a sex difference in the adaptive response of the myocardium to physiologic aging alone or together with cardiovascular diseases. Diastolic heart failure predominates in old women,82 and, although it is frequently observed in men, systolic and diastolic function is impaired more commonly in old men. The senescent male heart has an attenuated ability to sustain increases in pressure load, possibly mediated by the decrease in the number of myocytes early in life, which increases with age.8,87,88 Conversely, myocyte number remains constant in women up to nearly 90 years of age,88 and this difference may explain the higher incidence of systolic-diastolic heart failure in men.89
Myocardial Aging and CSCs
The concept of myocardial aging is complex, and this difficulty is dictated by the identification of parameters that define the consequences of time alone on the heart independent of cardiac and noncardiac pathologic abnormalities. Cardiac aging has been confounded by the notion that the heart is a postmitotic organ characterized by a predetermined number of myocytes, which is established at birth and preserved until the death of the organ and organism.2–5 According to this old paradigm, the generation of myocytes is restricted to the fetal neonatal heart, and organ hypertrophy in the adult occurs only by myocyte enlargement. Based on this premise, cellular, organ, and organism age coincide; at any given time, the heart is composed of a homogeneous population of myocytes of identical age. Because of this static view, aging has been construed as a time-dependent process that interacts with ischemic injury, hypertension, diabetes, and other disorders, which together define the clinical phenotype.75,82–84
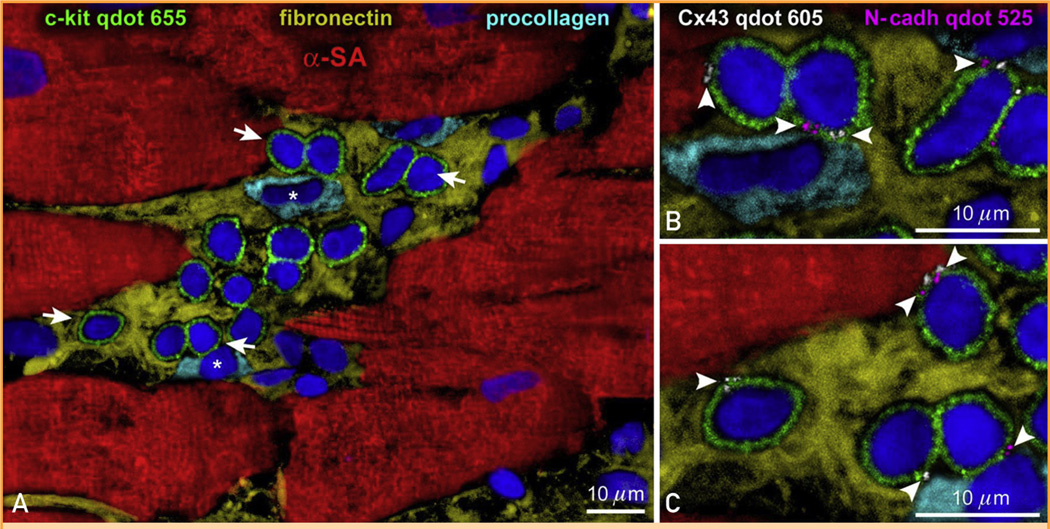
The discovery that stem cells live in the heart and differentiate into the various cardiac cell lineages has dramatically changed our understanding of myocardial biology.90–99 The CSCs are multipotent cells capable of generating cardiomyocytes and coronary vessels, providing the missing link between the identification of small dividing myocytes and the uncertainty concerning the origin of these repopulating cells (see references 10, 17, 100, and 101). Most importantly, this information has imposed a reconsideration of our view of cardiac homeostasis and myocardial aging. A new paradigm has emerged: the heart is a self-renewing organ characterized by resident CSCs stored in niches (Figure 4). The niches control the physiologic turnover of cardiac cells and the growth, migration, and commitment of CSCs that leave the niches, replacing dying cells in the myocardium.33,102–104 This information has imposed a reconsideration of myocardial aging.
FIGURE 4.
Cardiac niches in the human heart. A–C, Cluster of c-kit–positive cardiac stem cells (CSCs) (green). Arrows in A define the areas shown at higher magnification in B and C (magnification 1600×). Gap junctions (connexin 43 [C×43], white; arrowheads) and adherens junctions (N-cadherin [N-cadh], magenta; arrowheads) are illustrated. Connexin 43 and N-cadh are present between CSCs and myocytes (α-sarcomeric actin [SA], red) and fibroblasts (procollagen, light blue; asterisks). Fibronectin, yellow. qdot = quantum dots.
In the past, myocardial aging has been attributed to genetic modifications, accumulation of oxidative DNA damage, mutations in mitochondrial DNA, and telomeric dysfunction, triggering growth arrest, cellular senescence, and death.105–109 Although these determinants of organ aging are all valid, the cellular target in the heart may have changed. Aging effects on myocytes, smooth muscle cells, endothelial cells, and fibroblasts may be only an epiphenomenon dictated by CSC aging. Organ aging may reflect a process in which temporal alterations in CSC behavior determine the phenotypic properties of the myocardium. Myocardial aging may be regulated by time-dependent changes in the biology of CSCs. Growth defects at the level of the controlling cell, ie, the CSC, may perturb the structural integrity and function of the old heart. Old myocytes nested in proximity to CSCs may secrete a variety of inflammatory molecules and factors favoring cellular senescence and apoptosis, altering the physiologic behavior of stem cell niches in the myocardium.110 A typical example is provided by the enhanced synthesis and release of angiotensin II from hypertrophied cardiomyocytes,111–114 resulting in the activation of angiotensin II type 1 receptors present in CSCs.56,115 This process is commonly coupled with initiation of the apoptotic pathway. The effects of time on CSCs are critical for understanding the mechanisms conditioning CSC senescence, myocyte and vascular aging, and, ultimately, cardiac aging and organism lifespan.
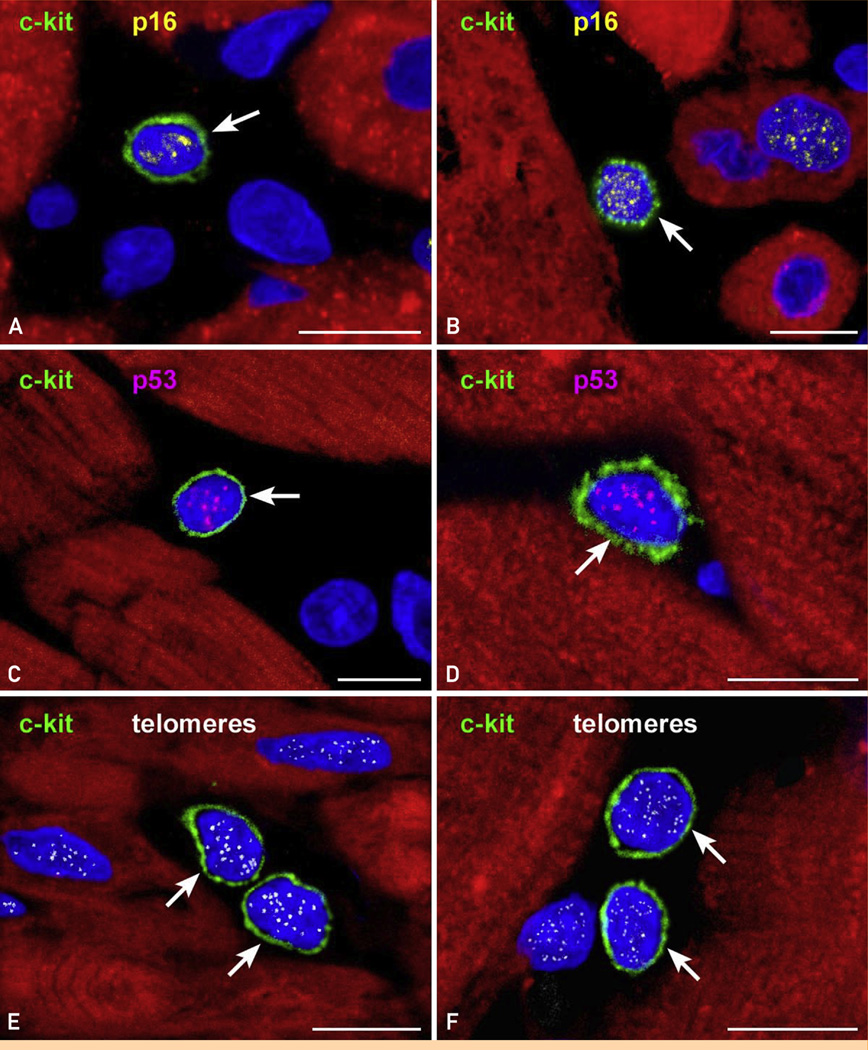
The telomere-telomerase axis is a determinant of cellular aging. Human CSCs (hCSCs) have telomeres of approximately 10 kilobase pairs (kbp), and with each cell division there is a loss of approximately 130 base pairs of telomeric DNA.92 Telomere length reflects the past replicative history and cumulative oxidative DNA damage occurring during the life cycle of the cell.105,107,109 Telomerase activity delays but cannot prevent telomere erosion, which is mediated by down-regulation of telomerase, formation of reactive oxygen species, and loss of telomere-related proteins.106 When telomere length reaches 1.5 to 2.0 kbp, replicative senescence and irreversible growth arrest occur.116 The presence of telomere-induced dysfunction foci in hCSCs is coupled with expression of the senescence-associated proteins p16INK4a and p53117 and, eventually, initiation of the apoptotic pathway (Figure 5).
FIGURE 5.
Cardiac stem cell (CSC) senescence. A–F, Two examples each of c-kit–positive CSCs (green) expressing (A and B) p16INK4a (yellow) and (C and D) p53 (magenta) are shown. E and F, Similarly, telomeres in c-kit–positive CSCs are illustrated in 2 examples by small white fluorescence dots. Arrows indicate c-kit–positive CSCs. Scale bars, 10 µm.
Growth activation of hCSCs with a wide range of telomere lengths leads to a progeny with distinct phenotypes. Chronological age results in telomere attrition of hCSCs, and these cells form myocytes that rapidly express p16INK4a.9 Daughter cells inherit the shortened telomeres of the maternal cells, and after a few rounds of division and terminal differentiation, they typically show an old cell phenotype.9,56 Telomere length in hCSCs and human myocytes shows a consistent pattern; if hCSCs exhibit long telomeres, the myocytes formed possess comparable telomere length. Conversely, hCSCs with short telomeres generate a myocyte progeny with severe telomere attrition. This phenomenon affects the aging female and male human heart; however, the proportion of hCSCs/myocytes with short telomeres is higher in men than in women, and the fraction of hCSCs/myocytes with long telomeres is larger in women than in men.9 Telomere length in hCSCs defines cellular aging independent of chronological age and organism lifespan. Oncogenic stress118may be viewed as an additional stimulus that accelerates senescence in amplifying myocytes. Oncogenic proliferative signals are coupled with several growth inhibitory responses generally seen with the induction of cellular aging and activation of the endogenous cell death pathway.119 Insulinlike growth factor 1 delays cardiac aging and rescues the infarcted myocardium experimentally,55,56,119,120 although it fails to initiate the division of terminally differentiated postmitotic myocytes. This parenchymal cell compartment may react with abortive mitosis121 or the formation of anaphase bridges.
Function of hCSCs
Human beings up to 104 years of age possess a significant number of hematopoietic stem cells with long telomeres and remarkable growth reserve that, however, are in a quiescent state.9 Conversely, most activated hCSCs in the senescent myocardium have short telomeres that are inherited by the specialized progeny. In the young healthy heart, the asymmetrical kinetics of stem cell growth efficiently preserves organ homeostasis. The structural and functional decline of the old and diseased heart may be coupled with defects in the hierarchical growth of the organ, suggesting that quantitative and qualitative alterations occur in resident hCSCs or in the pool of transit-amplifying cardiomyocytes. Both possibilities have been documented in stem cell–regulated organs.
The number of epithelial stem cells in the small intestine is gradually reduced with age,122 and the function of hematopoietic stem cells changes with time.123 Whether aging of the skin is conditioned by a decreased frequency of epidermal stem cells or by alterations in the kinetics of the transit-amplifying cell pool remains controversial.122,124 Similarly, the environmental and cell-autonomous mechanisms that maintain “young” hCSCs in a state of long-term quiescence remain to be identified. However, the existence of a pool of hCSCs with intact telomeres, 8 to 12 kbp, in senescent female and male hearts and in explanted hearts9,117 is of great clinical relevance. This category of hCSCs with high growth reserve is expected to generate a young myocyte progeny in the failing and senescent heart. Because each division of hCSCs results in the loss of approximately 130 base pairs of telomeric DNA,93 an extremely large number of cardiomyocytes can be formed by these cells before critical telomeric shortening and growth arrest occur. From a clinical perspective, the recognition that a subset of telomerase-competent hCSCs with long telomeres persists at all ages and with chronic heart failure has raised the possibility that autologous cell-based therapy may be feasible in patients with severe ventricular dysfunction. Recently, a method has been developed to isolate this compartment of functionally competent hCSCs from endomyocardial biopsies of patients undergoing cardiac transplant or left ventricular assist device implantation.125 After in vitro amplification, a clinically relevant number of hCSCs with high myogenic and vasculogenic potential was obtained. Expanded hCSCs possess a significant growth reserve as documented by the short population doubling time, high telomerase activity, and relatively long telomeres.
The phase 1 trial SCIPIO (Stem Cell Infusion in Patients with Ischemic cardiOmyopathy) involves the delivery of autologous c-kit–positive lineage-negative hCSCs for the treatment of severe chronic heart failure of ischemic origin.126,127 Patients with EF lower than 40% at 4 months after coronary artery bypass grafting were enrolled in the treatment and control groups. Treated patients received a single intracoronary infusion of 1 million autologous hCSCs. The primary end point was short-term treatment safety, and the secondary end point was efficacy. No hCSC-related adverse effects were reported. In 14 CSC-treated patients who were analyzed, EF increased from 30% to 38% at 4 months after infusion. In contrast, 7 control patients, during the corresponding interval, did not show any change in this functional parameter. The beneficial effects of CSCs were even more pronounced at 1 year. In 7 treated patients, infarct size decreased 24% and 30% at 4 and 12 months, respectively.126,127 These initial results are encouraging and warrant further studies.
CONCLUSION
The human heart is a highly dynamic organ regulated by a pool of resident hCSCs that modulate cardiac homeostasis and condition organ aging. Hopefully, recent findings will resolve the debate that has divided the scientific community into strong opponents and passionate supporters of the regenerative potential of the human heart. A common ground can now be found to translate this different perspective of cardiac biology into the development of novel strategies for the management of the human disease.
Abbreviations and Acronyms
- CSC
cardiac stem cell
- EF
ejection fraction
- hCSC
human cardiac stem cell
Footnotes
Individual reprints of this article and a bound reprint of the entire Symposium on Regenerative Medicine will be available for purchase from our website www.mayoclinicproceedings.org
REFERENCES
- 1.Anversa P, Leri A, Kajstura J. Cardiac regeneration. J Am Coll Cardiol. 2006;47(9):1769–1776. doi: 10.1016/j.jacc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Field LJ, Menasché P. Cell-based cardiac repair: reflections at the 10-year point. Circulation. 2005;112(20):3174–3183. doi: 10.1161/CIRCULATIONAHA.105.546218. [DOI] [PubMed] [Google Scholar]
- 3.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23(7):845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 4.Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu Rev Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 5.Hansson EM, Lindsay ME, Chien KR. Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell. 2009;5(4):364–377. doi: 10.1016/j.stem.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Yi BA, Wernet O, Chien KR. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest. 2010;120(1):20–28. doi: 10.1172/JCI40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbanek K, Quaini F, Tasca G, et al. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100(18):10440–10445. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urbanek K, Torella D, Sheikh F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102(24):8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kajstura J, Gurusamy N, Ogórek B, et al. Myocyte turnover in the aging human heart. Circ Res. 2010;107(11):1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 10.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109(8):941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123(1):62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Rota M, Hosoda T, De Angelis A, et al. The young mouse heart is composed of myocytes heterogeneous in age and function. Circ Res. 2007;101(4):387–399. doi: 10.1161/CIRCRESAHA.107.151449. [DOI] [PubMed] [Google Scholar]
- 13.Bernhard D, Laufer G. The aging cardiomyocyte: a mini-review. Gerontology. 2008;54(1):24–31. doi: 10.1159/000113503. [DOI] [PubMed] [Google Scholar]
- 14.Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol. 2011;57(1):9–17. doi: 10.1016/j.jacc.2010.08.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maier B, Gluba W, Bernier B, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18(3):306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosoda T, Rota M, Kajstura J, Leri A, Anversa P. Role of stem cells in cardiovascular biology. J Thromb Haemost. 2011;9(suppl 1):151–161. doi: 10.1111/j.1538-7836.2011.04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85(4):1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81(4):1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 19.Mori N, Horie Y, Nimura Y, Wolf R, Granger DN. Hepatic microvascular responses to ischemia-reperfusion in low-density lipoprotein receptor knockout mice. Am J Physiol Gastrointest Liver Physiol. 2000;279(6):G1257–G1264. doi: 10.1152/ajpgi.2000.279.6.G1257. [DOI] [PubMed] [Google Scholar]
- 20.Mori A, Hashino S, Kobayashi S, et al. Avascular necrosis in the femoral head secondary to bone marrow infarction in a patient with graft-versus-host disease after unrelated bone marrow transplantation. Ann Hematol. 2001;80(4):238–242. doi: 10.1007/s002770000253. [DOI] [PubMed] [Google Scholar]
- 21.Narayan S, Ezughah F, Standen GR, Pawade J, Kennedy CT. Idiopathic hypereosinophilic syndrome associated with cutaneous infarction and deep venous thrombosis. Br J Dermatol. 2003;148(4):817–820. doi: 10.1046/j.1365-2133.2003.05309.x. [DOI] [PubMed] [Google Scholar]
- 22.Francque S, Condat B, Asselah T, et al. Multifactorial aetiology of hepatic infarction: a case report with literature review. Eur J Gastroenterol Hepatol. 2004;16(4):411–415. doi: 10.1097/00042737-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Leong FT, Freeman LJ. Acute renal infarction. J R Soc Med. 2005;98(3):121–122. doi: 10.1258/jrsm.98.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackool RJ, Gittes GK, Longaker MT. Scarless healing: the fetal wound. Clin Plast Surg. 1998;25(3):357–365. [PubMed] [Google Scholar]
- 25.Ferguson MW, O’Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359(1445):839–850. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126(4):1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung A, Crombleholme TM, Keswani SG. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr. 2012;24(3):371–378. doi: 10.1097/MOP.0b013e3283535790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nye HL, Cameron JA, Chernoff EA, Stocum DL. Regeneration of the urodele limb: a review. Dev Dyn. 2003;226(2):280–294. doi: 10.1002/dvdy.10236. [DOI] [PubMed] [Google Scholar]
- 29.Hata S, Namae M, Nishina H. Liver development and regeneration: from laboratory study to clinical therapy. Dev Growth Differ. 2007;49(2):163–170. doi: 10.1111/j.1440-169X.2007.00910.x. [DOI] [PubMed] [Google Scholar]
- 30.Leri A, Kajstura J, Anversa P. Mechanisms of myocardial regeneration. Trends Cardiovasc Med. 2011;21(2):52–58. doi: 10.1016/j.tcm.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuchi K, Holdway JE, Werdich AA, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol. 2012;28:719–741. doi: 10.1146/annurev-cellbio-101011-155739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boni A, Urbanek K, Nascimbene A, et al. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105(40):15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey B, Izarra A, Alvarez R, et al. Cardiac stem cell genetic engineering using the alphaMHC promoter. Regen Med. 2009;4(6):823–833. doi: 10.2217/rme.09.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosoda T, Yasuzawa-Amano S, Amano K, et al. Defects in the c-kit-SCF system have profound effects on myocardial gene expression. Circulation. 2007;116:II_104. [Google Scholar]
- 37.Bastos Carvalho A, Carrillo-Infante C, D’Amario D, et al. Mutations of c-kit receptor are coupled with impaired growth and enhanced death of cardiac progenitor cells. Circulation. 2008;118:S_537–S_538. [Google Scholar]
- 38.Bailey B, Fransioli J, Gude NA, et al. Sca-1 knockout impairs myocardial and cardiac progenitor cell function. Circ Res. 2012;111(6):750–760. doi: 10.1161/CIRCRESAHA.112.274662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubin T, Pöling J, Kostin S, et al. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell. 2011;9(5):420–432. doi: 10.1016/j.stem.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Leri A, Kajstura J, Anversa P. Cardiac regeneration and aging. In: Rosenthal N, Harvey RP, editors. Heart Development and Regeneration. Vol II. Waltham, MA: Academic Press; 2010. pp. 951–980. [Google Scholar]
- 41.Leri A, Anversa P, Kajstura J. Stem cells and heart disease. In: Lanza R, Gearhart J, Hogan B, et al., editors. Essentials of Stem Cell Biology. 2nd ed. Waltham, MA: Academic Press; 2009. pp. 529–542. [Google Scholar]
- 42.Sugimoto K, Gordon SP, Meyerowitz EM. Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011;21(4):212–218. doi: 10.1016/j.tcb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Kragl M, Roensch K, Nüsslein I, et al. Muscle and connective tissue progenitor populations show distinct Twist1 and Twist3 expression profiles during axolotl limb regeneration. Dev Biol. 2013;373(1):196–204. doi: 10.1016/j.ydbio.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Gupta V, Poss KD. Clonally dominant cardiomyocytes direct heart morphogenesis. Nature. 2012;484(7395):479–484. doi: 10.1038/nature11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 46.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83(1):1–14. doi: 10.1161/01.res.83.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83(1):15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 48.Chien KR, Olson EN. Converging pathways and principles in heart development and disease: CV@CSH. Cell. 2002;110(2):153–162. doi: 10.1016/s0092-8674(02)00834-6. [DOI] [PubMed] [Google Scholar]
- 49.Oh H, Schneider MD. The emerging role of telomerase in cardiac muscle cell growth and survival. J Mol Cell Cardiol. 2002;34(7):717–724. doi: 10.1006/jmcc.2002.2018. [DOI] [PubMed] [Google Scholar]
- 50.Chien KR, Karsenty G. Longevity and lineages: toward the integrative biology of degenerative diseases in heart, muscle, and bone. Cell. 2005;120(4):533–544. doi: 10.1016/j.cell.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344(23):1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 52.Olivetti G, Ricci R, Anversa P. Hyperplasia of myocyte nuclei in long-term cardiac hypertrophy in rats. J Clin Invest. 1987;80(6):1818–1821. doi: 10.1172/JCI113278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chimenti C, Kajstura J, Torella D, et al. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93(7):604–613. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 54.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67(4):871–885. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- 55.Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94(4):514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez A, Rota M, Nurzynska D, et al. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102(5):597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 57.Murry CE, Lee RT. Development biology: turnover after the fallout. Science. 2009;324(5923):47–48. doi: 10.1126/science.1172255. [DOI] [PubMed] [Google Scholar]
- 58.Parmacek MS, Epstein JA. Cardiomyocyte renewal. N Engl J Med. 2009;361(1):86–88. doi: 10.1056/NEJMcibr0903347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 60.Rota M, Kajstura J, Hosoda T, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104(45):17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 62.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 63.Forester A. Handbuch der Speciellen Patholgischen Anatomie. Leipzig, Germany: 1852. [Google Scholar]
- 64.Zielonko G. Pathologisch anatomie und experiementelle studie uber hypertrophie des herzens. Virchows Arch Pathol Anat. 1875;62:29–55. [Google Scholar]
- 65.Wideroe S. Histologische studien uber die muskulatur des herzens. Virchows Arch Pathol Anat. 1911;240:190–196. [Google Scholar]
- 66.Aschoff L. Pathologische Anatomie. Leipzig, Germany: JA Barth; 1921. [Google Scholar]
- 67.Kaufmann E. Specielle Pathologische Anatomie. Berlin/Leipzig, Germany: 1922. [Google Scholar]
- 68.Karsner HT, Saphir O, Todd TW. The state of the cardiac muscle in hypertrophy and atrophy. Am J Pathol. 1925;1(4):351–372. [PMC free article] [PubMed] [Google Scholar]
- 69.Petersen RO, Baserga R. Nucleic acid and protein synthesis in cardiac muscle of growing and adult mice. Exp Cell Res. 1965;40(2):340–352. doi: 10.1016/0014-4827(65)90267-3. [DOI] [PubMed] [Google Scholar]
- 70.Morkin E, Ashford TP. Myocardial DNA synthesis in experimental cardiac hypertrophy. Am J Physiol. 1968;215(6):1409–1413. doi: 10.1152/ajplegacy.1968.215.6.1409. [DOI] [PubMed] [Google Scholar]
- 71.Grove D, Nair KG, Zak R. Biochemical correlates of cardiac hypertrophy, 3: changes in DNA content; the relative contributions of polyploidy and mitotic activity. Circ Res. 1969;25(4):463–471. doi: 10.1161/01.res.25.4.463. [DOI] [PubMed] [Google Scholar]
- 72.Grove D, Zak R, Nair KG, Aschenbrenner V. Biochemical correlates of cardiac hypertrophy, IV: observations on the cellular organization of growth during myocardial hypertrophy in the rat. Circ Res. 1969;25(4):473–485. doi: 10.1161/01.res.25.4.473. [DOI] [PubMed] [Google Scholar]
- 73.Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res. 1974;35(2) suppl II:17–26. [PubMed] [Google Scholar]
- 74.Anversa P, Olivetti G. Cellular basis of physiologic and pathologic myocardial growth. In: Page E, Fozzard HA, Solaro RJ, editors. Handbook of Physiology The Cardiovascular System, Section 2: The Heart. Vol 1. Bethesda, MD: American Physiological Society; 2001. pp. 75–144. [Google Scholar]
- 75.Anversa P, Rota M, Urbanek K, et al. Myocardial aging: a stem cell problem. Basic Res Cardiol. 2005;100(6):482–493. doi: 10.1007/s00395-005-0554-3. [DOI] [PubMed] [Google Scholar]
- 76.Aurigemma GP. Diastolic heart failure: a common and lethal condition by any name. N Engl J Med. 2006;355(3):308–310. doi: 10.1056/NEJMe068128. [DOI] [PubMed] [Google Scholar]
- 77.Kitzman DW, Gardin JM, Gottdiener JS, et al. Cardiovascular Health Study Research Group. Importance of heart failure with preserved systolic function in patients >or = 65 years of age. Am J Cardiol. 2001;87(4):413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 78.Aurigemma GP, Gaasch WH. Clinical practice: diastolic heart failure. N Engl J Med. 2004;351(11):1097–1105. doi: 10.1056/NEJMcp022709. [DOI] [PubMed] [Google Scholar]
- 79.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 80.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 81.Sanderson WC, Scherbov S. Average remaining lifetimes can increase as human populations age. Nature. 2005;435(7043):811–813. doi: 10.1038/nature03593. [DOI] [PubMed] [Google Scholar]
- 82.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics— 2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises, part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 84.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises, part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107(3):490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 85.Hadley EC, Lakatta EG, Morrison-Bogorad M, Warner HR, Hodes RJ. The future of aging therapies. Cell. 2005;120(4):557–567. doi: 10.1016/j.cell.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 86.Kajstura J, Rota M, Cappetta D, et al. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126(15):1869–1881. doi: 10.1161/CIRCULATIONAHA.112.118380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart: myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68(6):1560–1568. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 88.Olivetti G, Giordano G, Corradi D, et al. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26(4):1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 89.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 90.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 91.Linke A, Müller P, Nurzynska D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102(25):8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104(35):14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bearzi C, Leri A, Lo Monaco F, et al. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci U S A. 2009;106(37):15885–15890. doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Hosoda T, D’Amario D, Cabral-Da-Silva MC, et al. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci U S A. 2009;106(40):17169–17174. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100(21):12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pfister O, Mouquet F, Jain M, et al. CD31− but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97(1):52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 97.Tomita Y, Matsumura K, Wakamatsu Y, et al. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol. 2005;170(7):1135–1146. doi: 10.1083/jcb.200504061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oyama T, Nagai T, Wada H, et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176(3):329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115(7):896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 100.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113(11):1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 101.Anversa P, Leri A, Rota M, et al. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25(3):589–601. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 102.Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103(24):9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hosoda T, Zheng H, Cabral-da-Silva M, et al. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123(12):1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Goichberg P, Bai Y, D’Amario D, et al. The ephrin A1-EphA2 system promotes cardiac stem cell migration after infarction. Circ Res. 2011;108(9):1071–1083. doi: 10.1161/CIRCRESAHA.110.239459. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 106.Haigis MC, Yankner BA. The aging stress response. Mol Cell. 2010;40(2):333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102(11):1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blanpain C, Mohrin M, Sotiropoulou PA, Passegué E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8(1):16–29. doi: 10.1016/j.stem.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 109.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130(2):223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 110.Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J Cell Biol. 2011;193(2):257–266. doi: 10.1083/jcb.201010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leri A, Claudio PP, Li Q, et al. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest. 1998;101(7):1326–1342. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leri A, Liu Y, Li B, et al. Up-regulation of AT(1) and AT(2) receptors in postinfarcted hypertrophied myocytes and stretch-mediated apoptotic cell death. Am J Pathol. 2000;156(5):1663–1672. doi: 10.1016/S0002-9440(10)65037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leri A, Liu Y, Wang X, et al. Overexpression of insulin-like growth factor-1 attenuates the myocyte renin-angiotensin system in transgenic mice. Circ Res. 1999;84(7):752–762. doi: 10.1161/01.res.84.7.752. [DOI] [PubMed] [Google Scholar]
- 114.Leri A, Liu Y, Claudio PP, et al. Insulin-like growth factor-1 induces Mdm2 and down-regulates p53, attenuating the myocyte renin-angiotensin system and stretch-mediated apoptosis. Am J Pathol. 1999;154(2):567–580. doi: 10.1016/S0002-9440(10)65302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.D’Amario D, Cabral-Da-Silva MC, Zheng H, et al. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res. 2011;108(12):1467–1481. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994;91(21):9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cesselli D, Beltrami AP, D’Aurizio F, et al. Effects of age and heart failure on human cardiac stem cell function. Am J Pathol. 2011;179(1):349–366. doi: 10.1016/j.ajpath.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Urbanek K, Rota M, Cascapera S, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97(7):663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 120.Li Q, Ren J. Influence of cardiac-specific overexpression of insulin-like growth factor 1 on lifespan and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell. 2007;6(6):799–806. doi: 10.1111/j.1474-9726.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 121.Agah R, Kirshenbaum LA, Abdellatif M, et al. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest. 1997;100(11):2722–2728. doi: 10.1172/JCI119817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martin K, Kirkwood TB, Potten CS. Age changes in stem cells of murine small intestinal crypts. Exp Cell Res. 1998;241(2):316–323. doi: 10.1006/excr.1998.4001. [DOI] [PubMed] [Google Scholar]
- 123.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102(26):9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10(3):207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.D’Amario D, Fiorini C, Campbell PM, et al. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108(7):857–861. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.Chugh AR, Beache GM, Loughran JH, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11) suppl 1:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]