Abstract
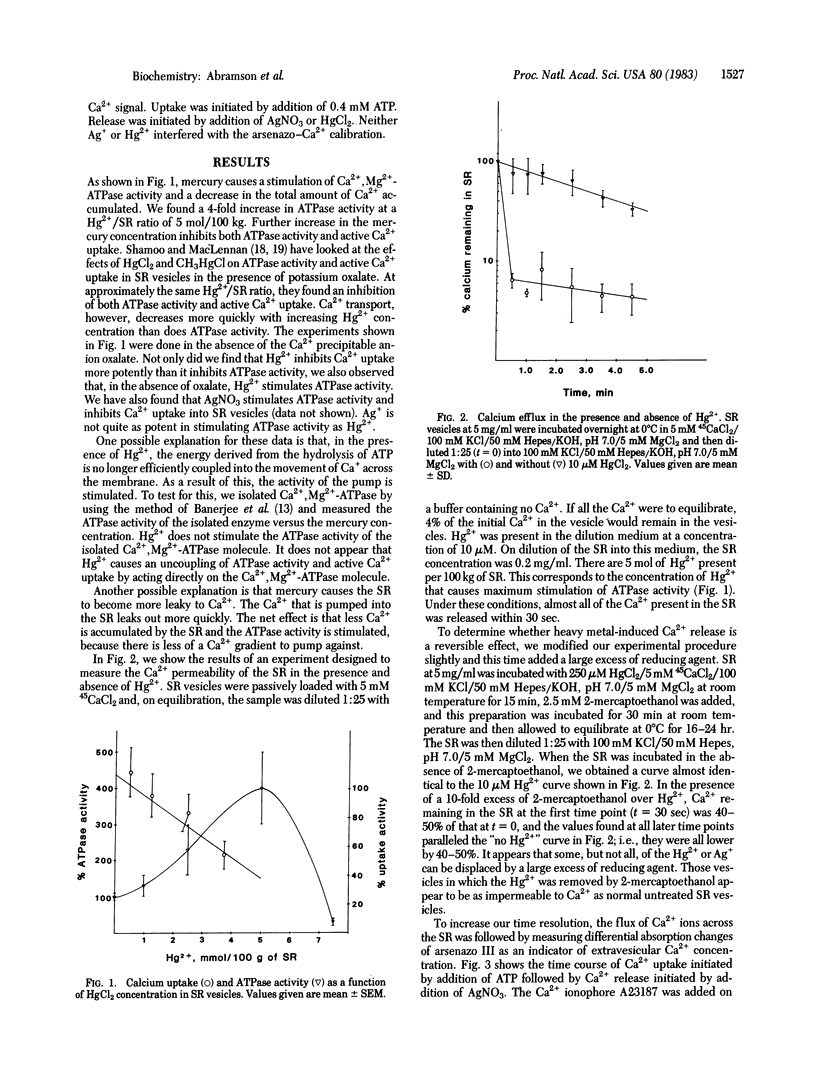
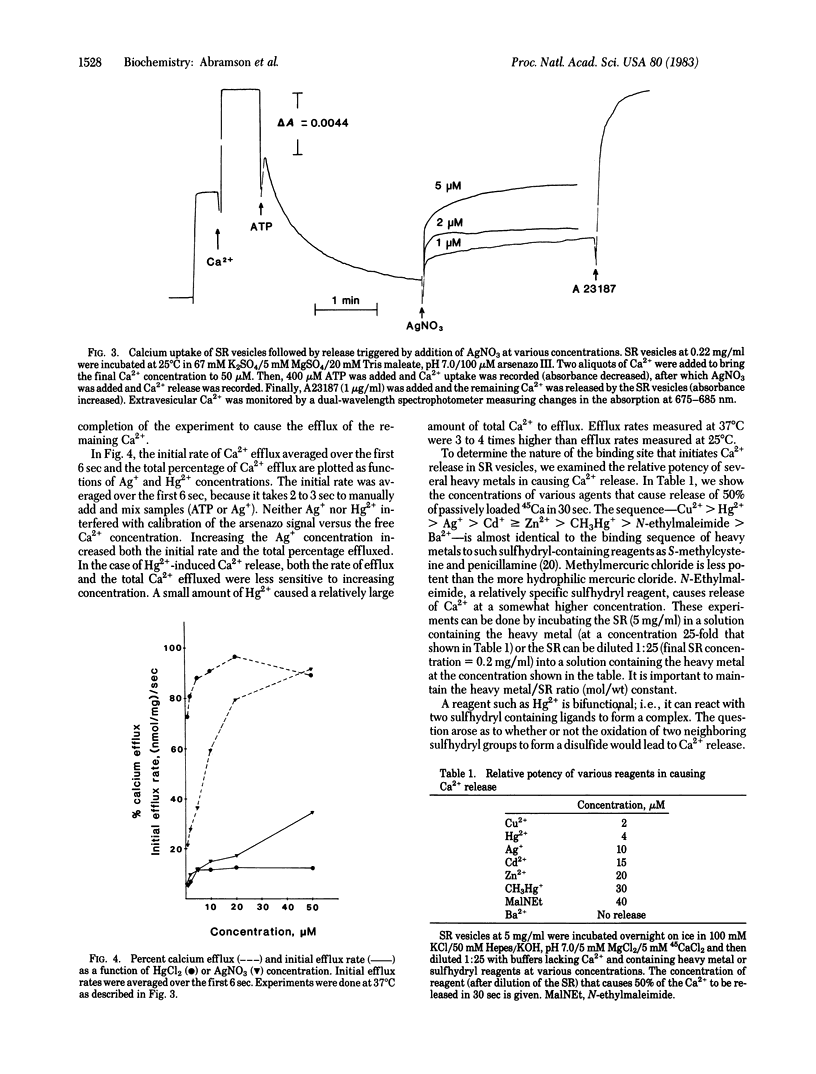
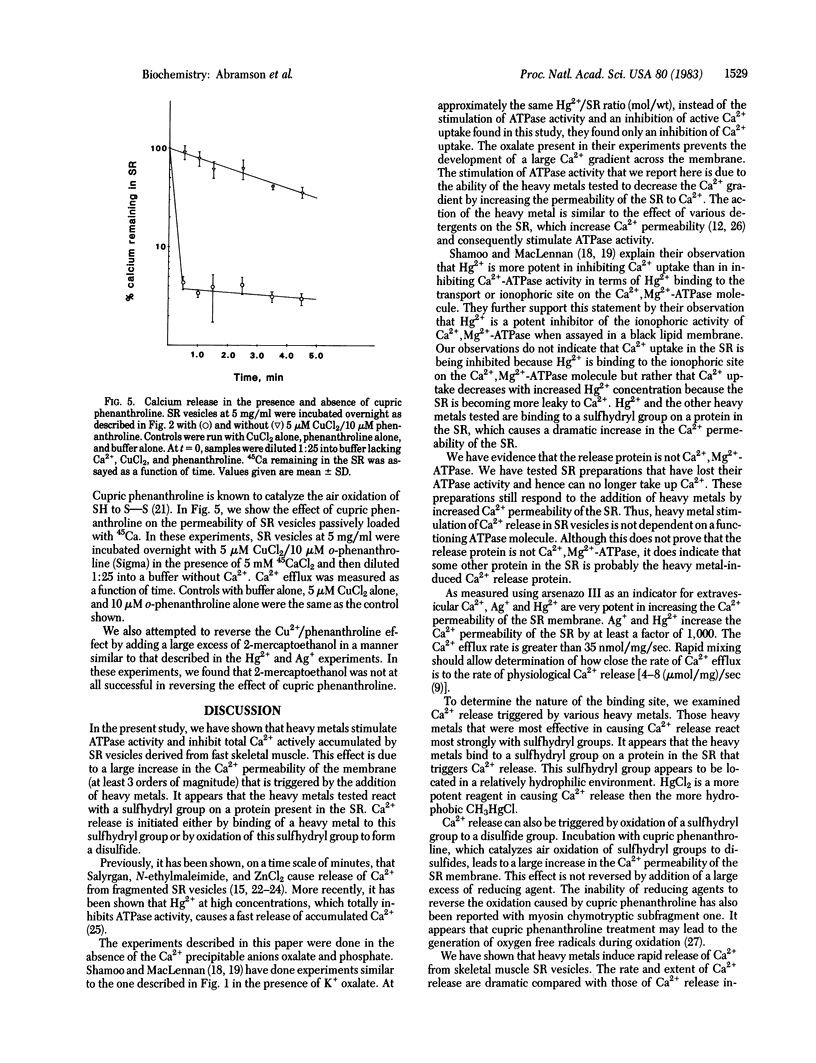
Micromolar concentrations of mercury, silver, and other reagents known to react with sulfhydryl groups are shown to stimulate ATPase activity and inhibit active calcium uptake in sarcoplasmic reticulum vesicles derived from rabbit fast skeletal muscle. These effects are caused by a dramatic increase in the calcium permeability of the sarcoplasmic reticulum. Measurements of Ca2+ permeability were made using both isotopes and by spectrophotometric techniques using the Ca2+ indicator arsenazo III. Air oxidation of a sulfhydryl group to a disulfide group also leads to a large increase in the calcium permeability of the sarcoplasmic reticulum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. J., Shamoo A. E. Anionic detergents as divalent cation ionophores across black lipid membranes. J Membr Biol. 1979 Nov 30;50(3-4):241–255. doi: 10.1007/BF01868891. [DOI] [PubMed] [Google Scholar]
- Banerjee R., Epstein M., Kandrach M., Zimniak P., Racker E. A new method of preparing Ca2+-ATPase from sarcoplasmic reticulum: extraction with octylglucoside. Membr Biochem. 1979;2(3-4):283–296. doi: 10.3109/09687687909063868. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Horowicz P. Fluorescence intensity changes associated with contractile activation in frog muscle stained with Nile Blue A. J Physiol. 1975 Apr;246(3):709–735. doi: 10.1113/jphysiol.1975.sp010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Legallais V., Sorge J., Graham N. A versatile time-sharing multichannel spectrophotometer, reflectometer, and fluorometer. Anal Biochem. 1975 Jun;66(2):498–514. doi: 10.1016/0003-2697(75)90617-x. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Inesi G. The use of quench reagents for resolution of single transport cycles in sarcoplasmic reticulum. J Biol Chem. 1979 Oct 25;254(20):10370–10377. [PubMed] [Google Scholar]
- Duggan P. F., Martonosi A. Sarcoplasmic reticulum. IX. The permeability of sarcoplasmic reticulum membranes. J Gen Physiol. 1970 Aug;56(2):147–167. doi: 10.1085/jgp.56.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium release from the sarcoplasmic reticulum. Circ Res. 1977 Feb;40(2):119–129. doi: 10.1161/01.res.40.2.119. [DOI] [PubMed] [Google Scholar]
- Fairhurst A. S., Hasselbach W. Calcium efflux from a heavy sarcotubular fraction. Effects of ryanodine, caffeine and magnesium. Eur J Biochem. 1970 Apr;13(3):504–509. doi: 10.1111/j.1432-1033.1970.tb00953.x. [DOI] [PubMed] [Google Scholar]
- Kasai M., Miyamoto Depolarization-induced calcium release from sarcoplasmic reticulum fragments. II. Release of calcium incorporated with ATP. J Biochem. 1976 May;79(5):1067–1076. doi: 10.1093/oxfordjournals.jbchem.a131148. [DOI] [PubMed] [Google Scholar]
- Kasai M., Miyamoto H. Depolarization induced calcium release from sarcoplasmic reticulum membrane fragments by changing ionic environment. FEBS Lett. 1973 Aug 15;34(2):299–301. doi: 10.1016/0014-5793(73)80816-6. [DOI] [PubMed] [Google Scholar]
- Kobashi K. Catalytic oxidation of sulfhydryl groups by o-phenanthroline copper complex. Biochim Biophys Acta. 1968 May;158(2):239–245. doi: 10.1016/0304-4165(68)90136-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTONOSI A., FERETOS R. SARCOPLASMIC RETICULUM. I. THE UPTAKE OF CA++ BY SARCOPLASMIC RETICULUM FRAGMENTS. J Biol Chem. 1964 Feb;239:648–658. [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- Meissner G., McKinley D. Permeability of sarcoplasmic reticulum membrane. The effect of changed ionic environments on Ca2+ release. J Membr Biol. 1976 Dec 25;30(1):79–98. doi: 10.1007/BF01869661. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Racker E. Calcium-induced calcium release at terminal cisternae of skeletal sarcoplasmic reticulum. FEBS Lett. 1981 Oct 26;133(2):235–238. doi: 10.1016/0014-5793(81)80513-3. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Racker E. Mechanism of calcium release from skeletal sarcoplasmic reticulum. J Membr Biol. 1982;66(3):193–201. doi: 10.1007/BF01868494. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Endo M. Release of calcium induced by 'depolarisation' of the sarcoplasmic reticulum membrane. Nat New Biol. 1973 Dec 19;246(155):216–218. doi: 10.1038/newbio246216a0. [DOI] [PubMed] [Google Scholar]
- Nakamaru Y., Schwartz A. Possible control of intracellular calcium metabolism by [H+]: sarcoplasmic reticulum of skeletal and cardiac muscle. Biochem Biophys Res Commun. 1970 Nov 25;41(4):830–836. doi: 10.1016/0006-291x(70)90157-9. [DOI] [PubMed] [Google Scholar]
- Ohnishi S. T. Calcium-induced calcium release from fragmented sarcoplasmic reticulum. J Biochem. 1979 Oct;86(4):1147–1150. doi: 10.1093/oxfordjournals.jbchem.a132609. [DOI] [PubMed] [Google Scholar]
- Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974 Aug;60(2):489–504. doi: 10.1016/0003-2697(74)90259-0. [DOI] [PubMed] [Google Scholar]
- Shamoo A. E., MacLennan D. H. Separate effects of mercurial compounds on the ionophoric and hydrolytic functions of the (Ca++ +Mg++)-ATPase of sarcoplasmic reticulum. J Membr Biol. 1975 Dec 4;25(1-2):65–74. doi: 10.1007/BF01868568. [DOI] [PubMed] [Google Scholar]
- Shamoo A. E., Maclennan D. H., Elderfrawi M. E. Differential effects of mercurial compounds on excitable tissues. Chem Biol Interact. 1976 Jan;12(1):41–52. doi: 10.1016/0009-2797(76)90065-x. [DOI] [PubMed] [Google Scholar]
- Shoshan V., MacLennan D. H., Wood D. S. A proton gradient controls a calcium-release channel in sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4828–4832. doi: 10.1073/pnas.78.8.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. A., Werber M. M., Yount R. G. Inactivation of myosin subfragment one by cobalt(II)/cobalt(III) phenanthroline complexes. 2. Cobalt chelation of two critical SH groups. Biochemistry. 1979 Oct 30;18(22):4800–4805. doi: 10.1021/bi00589a006. [DOI] [PubMed] [Google Scholar]


