The structure of the C-terminal domain of the CarD protein from M. tuberculosis is reported.
Keywords: Mycobacterium tuberculosis CarD, Thermus aquaticus RNAP β subunit, CarD binding analysis
Abstract
The CarD protein is highly expressed in mycobacterial strains under basal conditions and is transcriptionally induced during multiple types of genotoxic stress and starvation. The CarD protein binds the β subunit of RNA polymerase and influences gene expression. The disruption of interactions between CarD and the β subunit of RNA polymerase has a significant effect on mycobacterial survival, resistance to stress and pathogenesis. To understand the structure of CarD and its interaction with the β subunit of RNA polymerase, Mycobacterium tuberculosis CarD (MtbCarD) and the Thermus aquaticus RNA polymerase β subunit were recombinantly expressed and purified. Secondary-structure analysis using circular-dichroism spectroscopy indicated that MtbCarD contains ∼60% α-helix, ∼7% β-sheet and ∼33% random-coil structure. The C-terminal domain of MtbCarD (CarD83–161) was crystallized and its X-ray structure was determined at 2.1 Å resolution. CarD83–161 forms a distorted Y-shaped structure containing bundles of three helices connected by a loop. The residues forming the distorted Y-shaped structure are highly conserved in CarD sequences from other mycobacterial species. Comparison of the CarD83–161 structure with the recently determined full-length M. tuberculosis and T. thermophilus CarD crystal structures revealed structural differences in residues 141–161 of the C-terminal domain of the CarD83–161 structure. The structural changes in the CarD83–161 structure occurred owing to proteolysis and crystallization artifacts.
1. Introduction
According to World Health Organization statistics, 30% of the world population is infected by Mycobacterium tuberculosis (Mtb) and it causes 1.4 million deaths per year (World Health Organization, 2011 ▶). There is an essential requirement to develop drugs and vaccines against multidrug-resistant, extensively drug-resistant and totally drug-resistant strains of mycobacteria. During a study of the stress response of mycobacteria, it was found that the carD gene was transcriptionally induced under multiple types of stress (Stallings et al., 2009 ▶). On transient knockout of the CarD gene in mycobacteria, the cells become sensitive to killing by starvation, reactive oxygen species and ciprofloxacin stresses (Stallings et al., 2009 ▶). M. tuberculosis bacilli with a depleted carD gene did not replicate and persist in mice, and a global change in the transcriptional profiles of mycobacteria was observed in a microanalysis experiment (Stallings et al., 2009 ▶).
Mycobacterial CarD protein is required for the efficient control of rRNA transcription by (p)ppGpp (Stallings et al., 2009 ▶). CarD is a functional homologue of DksA and interacts directly with the RNA polymerase (RNAP) β subunit (Stallings et al., 2009 ▶). A study of mycobacterial strains with attenuated CarD interactions with the RNAP β subunit indicated that this interaction is required for resistance to oxidative stress and for viability and is not required for fluoroquinolone resistance (Weiss et al., 2012 ▶). A complete phenotype was not observed on weakening the interaction of MtbCarD with the MtbRNAP β subunit (Weiss et al., 2012 ▶).
MtbCarD contains 162 residues with a molecular mass of ∼17.9 kDa. The N-terminal region of MtbCarD contains a TRCF-RID module and interacts with the N-terminus of the MtbRNAP β subunit. Immunoprecipitation of CarD from M. smegmatis cell lysate indicated that the α, β and β′ domains of the mycobacterial RNA polymerase bind directly to CarD. The C-terminal region of MtbCarD contains a leucine zipper-like fold and is essential for mycobacterial viability, as shown in a gene-deletion experiment (Stallings et al., 2009 ▶). Recently, the crystal structure of full-length M. tuberculosis CarD in complex with RNAP β lobes (Gulten & Sacchettini, 2013 ▶; PDB entry 4kbm) and the crystal structure of Thermus thermophilus CarD (Srivastava et al., 2013 ▶; PDB entry 4l5g) have been determined.
Mycobacterial CarD binds directly to RNA polymerase and influences gene expression (Stallings et al., 2009 ▶). To understand the MtbCarD structure and its interaction with the T. aquaticus RNAP (TaqRNAP) β subunit, we expressed and purified the MtbCarD and TaqRNAP β subunit proteins. We performed CD spectroscopy to identify the secondary-structure content of MtbCarD. The crystal structure of the CarD83–161 domain was analyzed and compared with recently determined X-ray structures of M. tuberculosis and T. thermophilus CarD proteins.
2. Materials and methods
2.1. MtbCarD expression and purification
The gene encoding residues Met1–Ser162 of CarD (accession No. NP_218100) was amplified using genomic DNA of M. tuberculosis H37Rv strain by polymerase chain reaction (PCR) and inserted into pET-28a(+) expression vector (Novagen). The forward primer 5′-GATCCATATGATGATTTTCAAGGTCGGA-3′ and the reverse primer 5′-CATGAAGCTTTCAAGACGCGGCGGCTAA-3′ were used in the polymerase chain reaction. The amplified PCR product was digested and ligated into pET-28a(+) expression vector using NdeI and HindIII restriction sites. The CarD plasmid was transformed into Escherichia coli BL21(DE3) cells for protein expression.
The E. coli cells were grown in 2 l Luria–Bertani medium supplemented with 50 µg ml−1 kanamycin at 37°C until the OD600 reached 0.6–0.7. The culture was induced with 250 µM IPTG at 37°C and grown for a further 3 h. The cells were harvested by centrifugation at 10 000g and resuspended in 100 ml lysis buffer consisting of 20 mM HEPES pH 7.0, 100 mM NaCl, 1 mM benzamidine–HCl, 0.1% Triton X-100, 5% glycerol, 1 mM phenylmethylsulfonyl fluoride, 0.2 mg ml−1 lysozyme. The cells were kept on ice for a further 1 h and disrupted by sonication. The cell lysate was centrifuged at 16 000g for 20 min and the supernatant was collected.
For Ni–NTA affinity chromatography, the supernatant was mixed with pre-equilibrated Ni–NTA resin and incubated for 2 h at 4°C using a 360° rocker. The resin was washed with buffer consisting of 20 mM HEPES pH 7.0, 500 mM NaCl, 1 mM benzamidine–HCl, 5% glycerol, 1 mM phenylmethylsulfonyl fluoride, 40 mM imidazole. The MtbCarD protein was eluted with buffer consisting of 20 mM HEPES pH 7.0, 150 mM NaCl, 1 mM benzamidine–HCl, 5% glycerol, 1 mM phenylmethylsulfonyl fluoride, 250 mM imidazole. The eluted MtbCarD fractions were pooled, concentrated and loaded onto a gel-filtration column (HiLoad 16/60 Superdex 75 pg, GE Healthcare). The column was pre-equilibrated with buffer consisting of 20 mM HEPES pH 7.0, 150 mM NaCl. The peak fractions were pooled and concentrated to 10–12 mg ml−1 using a centrifugal filter device (Millipore, USA). The protein was stored at 4°C. The final recombinant MtbCarD contained 183 residues with a molecular mass of 20.2 kDa [an extra 21 residues at the N-terminus (MGSSHHHHHHSSGLVPRGSHM containing a thrombin cleavage site) and 162 residues of CarD]. The purity of the recombinant MtbCarD was analyzed by SDS–PAGE and mass spectrometry. The protein concentration was estimated using the Bradford technique (Bradford, 1976 ▶).
2.2. TaqRNAP β subunit expression and purification
The pET-21c plasmid containing the TaqRNAP β subunit gene was transformed into E. coli BL21(DE3) cells for protein expression. A single colony was inoculated in 5 l Luria–Bertani medium supplemented with 100 µg ml−1 ampicillin. The culture was grown at 37°C until the OD600 reached 0.6–0.7. The culture was induced with 1 mM IPTG at 37°C and grown for a further 3 h. The cells were harvested by centrifugation at 10 000g and the pellet was resuspended in 100 ml lysis buffer consisting of 20 mM Tris–HCl pH 8.0, 500 mM NaCl, 1 mM benzamidine–HCl, 0.1% Triton X-100, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 0.5 mg ml−1 lysozyme. The cells were kept on ice for a further 1 h and disrupted by sonication. The cell lysate was centrifuged at 16 000g for 20 min and the supernatant was collected.
For Ni–NTA affinity chromatography, the supernatant was mixed with pre-equilibrated Ni–NTA resin and incubated for 2 h at 4°C using a 360° rocker. The mixture was loaded into an empty column and the resin was washed with buffer consisting of 20 mM Tris–HCl pH 8.0, 500 mM NaCl, 1 mM benzamidine–HCl, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 45 mM imidazole. The protein was eluted in buffer consisting of 20 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 mM benzamidine–HCl, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 250 mM imidazole. The eluted protein fractions were pooled, concentrated and loaded onto a gel-filtration column (HiLoad 16/60 Superdex 200 pg, GE Healthcare). The column was pre-equilibrated with buffer consisting of 20 mM Tris–HCl pH 8.0, 150 mM NaCl, 10% glycerol, 5 mM β-mercaptoethanol. The peak fractions were pooled and concentrated to 5 mg ml−1 using a centrifugal filter device (Millipore, USA). The protein was stored at 4°C. The purity of the TaqRNAP β subunit was analyzed by SDS–PAGE and mass spectrometry. The protein concentration was estimated using the Bradford method (Bradford, 1976 ▶).
2.3. Circular-dichroism (CD) analysis
CD measurements were recorded using a Chirascan CD spectropolarimeter (Applied Photophysics) with a water bath to maintain a constant temperature. The MtbCarD was diluted to 0.1 mg ml−1 in 10 mM Tris–HCl buffer pH 8.0 and loaded into a 0.1 cm quartz cuvette. The blank for all experiments was 10 mM Tris–HCl buffer pH 8.0. The final spectrum was the average of three sequential scans. The CD data were converted to mean residue ellipticities (in deg cm2 dmol−1). The DichroWeb server (Whitmore & Wallace, 2004 ▶) was used to estimate the amount of secondary structure from the CD spectra and estimated ∼60% α-helix, ∼7% β-sheet and ∼33% random-coil structure.
2.4. Crystallization and heavy-atom derivatization
Selenomethionine-substituted MtbCarD protein was prepared using the following protocol. Complete selenomethionine medium was prepared by mixing the base medium with nutrient mixture from Molecular Dimensions (MD 12-501). The base medium was autoclaved and filter-sterilized nutrient mixture was added to the base medium to prepare complete selenomethionine medium. 40 mg seleno-dl-methionine from Sigma–Aldrich was added to 1 l selenomethionine medium. The primary culture was grown overnight at 37°C and the obtained cell pellet was resuspended in complete selenomethionine medium. It was centrifuged again and resuspended in complete selenomethionine medium. This culture was used as a starting culture for large-scale purification of selenomethionine-derivatized MtbCarD. The expression and purification procedure used for selenomethionine-derivatized MtbCarD was similar to that described for native MtbCarD.
Both native and selenomethionine-derivative MtbCarD proteins were used for crystallization experiments. Initial crystallization conditions were obtained using Crystal Screen and Crystal Screen 2 from Hampton Research and the JCSG-plus screen from Molecular Dimensions. The sitting-drop vapour-diffusion technique was used in initial crystallization trials at 4°C. In each trial, 1 µl protein solution was mixed with 1 µl reservoir solution and equilibrated against 100 µl reservoir solution. Needle-shaped crystals of MtbCarD and selenomethionine-derivatized MtbCarD appeared after 4–5 d in several crystallization conditions. Further optimization of the crystallization conditions yielded the best diffracting crystals of MtbCarD using a reservoir consisting of 0.2 M MgSO4, 16%(w/v) PEG 4000, 30%(v/v) ethylene glycol.
Other heavy-atom derivatives of MtbCarD were prepared in which native MtbCarD crystals were soaked in reservoir solution containing the following heavy-atom compounds: (i) 500 mM NaI for 2 min, (ii) 10 mM K2PtCl4 for 24 h and (iii) 5 mM HgCl2 for 24 h.
2.5. Intensity data collection and X-ray structure solution
For intensity data collection, single crystals of MtbCarD were cooled directly in liquid nitrogen, as 30% ethylene glycol acts as a good cryoprotectant. The native CarD crystal diffracted to 2.1 Å resolution and an X-ray intensity data set was collected on the BM14 beamline at the European Synchrotron Radiation Facility, Grenoble, France. The selenomethionine-derivative MtbCarD crystal diffracted to 6.0 Å resolution and a single anomalous diffraction data set at the Se peak was collected at 6.0 Å resolution (data not shown).
A single anomalous diffraction data set was collected from an NaI-soaked MtbCarD crystal using Cu Kα radiation (λ = 1.5418 Å) at the X-ray diffraction facility (AIRF) of Jawaharlal Nehru University (JNU), New Delhi, India. Merging of raw data sets was performed using iMosflm (Battye et al., 2011 ▶). The scaling and processing of data sets were performed using CCP4 (Winn et al., 2011 ▶). The intensity data-collection and processing statistics are given in Table 1 ▶. Iodide sites were obtained using single anomalous diffraction data collected from an NaI-soaked crystal using SHELXD from the HKL2MAP suite (Pape & Schneider, 2004 ▶). Initial phases were obtained using the AutoSol program and the model was built using AutoBuild from the PHENIX suite (Adams et al., 2010 ▶). The AutoBuild program traced only the C-terminal residues Thr83–Ala161 of MtbCarD, and the electron density for the N-terminal residues 1–82 was missing. The MtbCarD crystals were dissolved and the protein was analyzed by SDS–PAGE. The protein showed a single band of ∼9 kDa (the molecular mass of full-length CarD is ∼20.2 kDa). To identify the cleavage site in the crystallized MtbCarD protein, we dissolved the MtbCarD crystals and performed N-terminal sequencing of the protein obtained from these crystals (Supplementary Table S11). The sequencing result indicated the first seven residues to be APHTEEP, e.g. residues 76–82 of MtbCarD. This indicates that residues 76–82 were disordered in our MtbCarD83–161 crystal structure and could not be traced. The CarD83–161 structure was refined using PHENIX (Adams et al., 2010 ▶) and the model was rebuilt using Coot (Emsley & Cowtan, 2004 ▶). Figures were generated by PyMOL (DeLano, 2002 ▶).
Table 1. Intensity data-collection and refinement statistics for CarD83161 .
Values in parentheses are for the highest resolution shell.
| Data set | Native | Iodide-soaked |
|---|---|---|
| Space group | P43212 | P43212 |
| Temperature (K) | 100 | 100 |
| Wavelength () | 0.97625 | 1.5418 |
| X-ray source | BM14, ESRF | AIRF, JNU |
| Resolution () | 50.002.15 (2.192.15) | 60.932.75 (2.902.75) |
| Unit-cell parameters () | a = b = 60.95, c = 59.47 | a = b = 60.93, c = 59.37 |
| Observed reflections | 179423 | 53716 |
| Unique reflections | 6559 (334) | 3111 (349) |
| Completeness (%) | 99.9 (100) | 96.8 (77.8) |
| R merge † (%) | 8.0 (50.3) | 13.6 (48.4) |
| Average I/(I) | 10.3 (7.9) | 19.5 (6.3) |
| Multiplicity | 27.4 (28.6) | 17.3 (17.2) |
| Wilson B factor (2) | 43.81 | |
| Molecules in asymmetric unit | 1 | 1 |
| V M (3Da1) | 2.87 | 2.87 |
| Solvent content (%) | 57.2 | 57.2 |
| Refinement | ||
| Resolution () | 30.472.14 | |
| R work/R free ‡ | 0.21/0.25 | |
| Protein atoms | 616 | |
| Water atoms | 15 | |
| R.m.s.d., bonds () | 0.007 | |
| R.m.s.d., angles () | 0.866 | |
| Average B factor (2) | ||
| Protein | 49.70 | |
| Water | 50.40 | |
| Ramachandran plot (%) | ||
| Favoured | 100 | |
| Allowed | 0 | |
| Generously allowed | 0 | |
| Forbidden | 0 | |
| PDB code | 4kmc | |
R
merge = 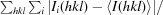
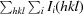 , where I
i(hkl) is the ith intensity measurement of reflection hkl and I(hkl) is the average intensity of that reflection.
, where I
i(hkl) is the ith intensity measurement of reflection hkl and I(hkl) is the average intensity of that reflection.
R = 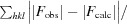
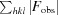 .
.
3. Results and discussion
3.1. MtbCarD exists as a monomer in solution
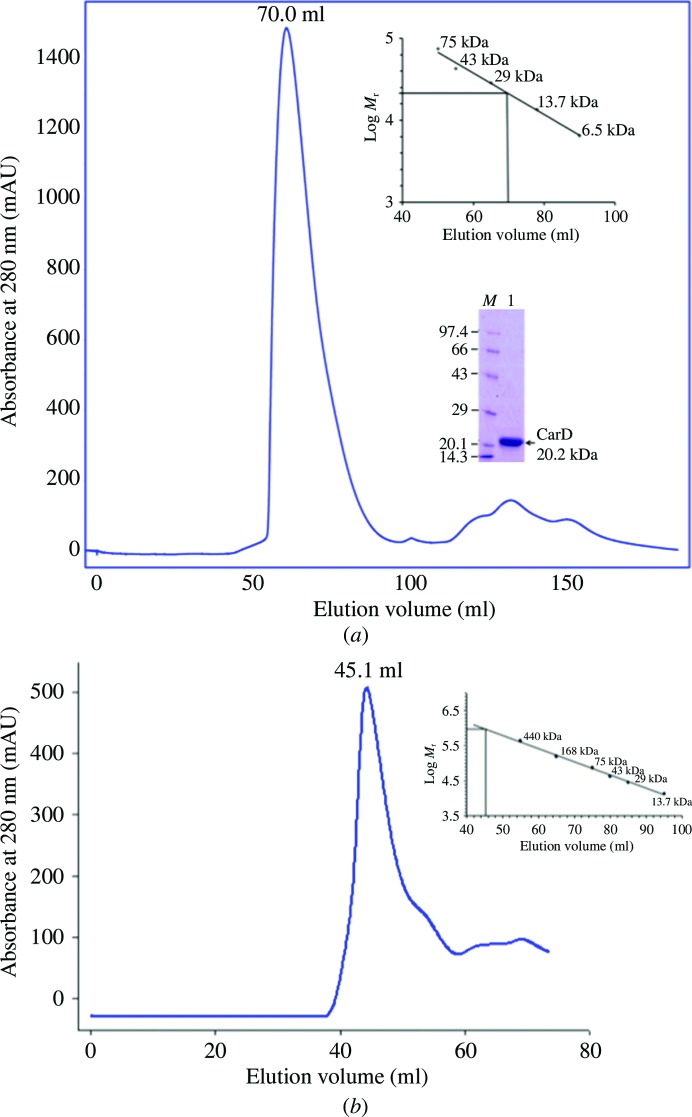
Recombinant MtbCarD (183 residues, molecular mass ∼20.2 kDa) and TaqRNAP β subunit (1119 residues, molecular mass ∼124.7 kDa) were expressed and purified as described in §2. The recombinant MtbCarD eluted as a monomer from the size-exclusion column and showed a single peak on SDS–PAGE (Fig. 1 ▶). Single crystals of MtbCarD were grown using the hanging-drop vapour-diffusion technique and diffracted to 2.1 Å resolution. However, the MtbCarD protein degraded during crystallization and the X-ray intensity data yielded the structure of only the C-terminal residues Thr83–Ala161 of MtbCarD. SDS–PAGE analysis of the protein obtained from the MtbCarD83–161 crystals indicated a molecular mass of ∼9 kDa. We dissolved the MtbCarD83–161 crystals and performed N-terminal sequencing of the obtained protein (Supplementary Table S1). This indicated that the protein is cleaved after residue 75 and that residues 76–162 of MtbCarD are present in the crystallized protein. The recombinant TaqRNAP β subunit aggregated and eluted in the void volume of the Superdex 200 (16/60) column (Fig. 1 ▶).
Figure 1.
Size-exclusion chromatography of MtbCarD and the TaqRNAP β subunit. (a) Size-exclusion chromatogram and SDS–PAGE of full-length MtbCarD protein. MtbCarD eluted as a monomer from a Superdex 75 (16/60) column. (b) Size-exclusion chromatogram of purified TaqRNAP β subunit. The TaqRNAP β subunit eluted as an aggregate from a Superdex 200 (16/60) column. The calculated molecular mass of each protein is denoted.
3.2. Secondary-structure content of MtbCarD
Far-UV CD spectroscopy was used to estimate the secondary-structure content of MtbCarD. The CD data were deconvoluted using the DichroWeb server (Whitmore & Wallace, 2004 ▶) and the percentages of α-helix, β-sheet and random-coil structure were evaluated. The CD data predicted ∼60% α-helix, ∼7% β-sheet and ∼33% random-coil structure in full-length MtbCarD. These values were close to the secondary-structure content observed in the crystal structure of MtbCarD (Gulten & Sacchettini, 2013 ▶) and from various theoretical calculations on MtbCarD (∼59% α-helix, ∼10.5% β-sheet and ∼30.5% random coil). The secondary-structure content of MtbCarD obtained using PSIPRED (Jones, 1999 ▶) analysis is shown in Supplementary Fig. S1.
3.3. Crystal structure of CarD83–161
We set up crystallization trials using full-length MtbCarD protein. However, the protein degraded during crystallization and the X-ray intensity data obtained yielded the structure of only residues Thr83–Ala161 of MtbCarD (CarD83–161). The rhombohedral-shaped crystals of CarD83–161 grew in mother liquor consisting of 20%(w/v) PEG 4000, 0.2 M MgSO4, 30% ethylene glycol to dimensions of approximately 0.5 × 0.3 × 0.1 mm. The phases for the CarD83–161 structure were obtained by the single anomalous dispersion (SAD) technique using an iodide SAD data set collected at 2.8 Å resolution. The CarD83–161 structure obtained from the iodide SAD data was placed in the native CarD data set collected at 2.1 Å resolution and refined to an R factor of 0.21 and an R free of 0.25. All residues of CarD83–161 fall in the favourable region of the Ramachandran plot.
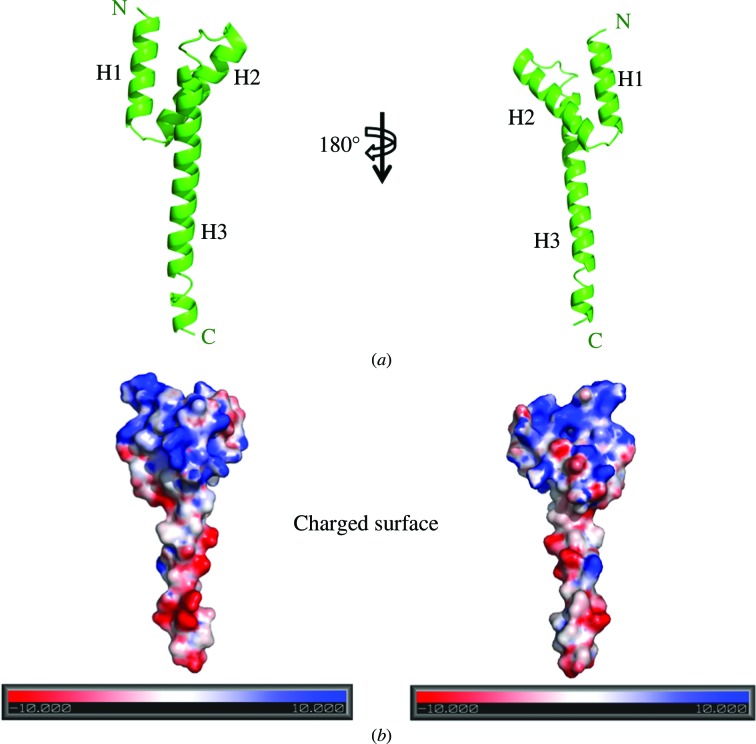
CarD83–161 forms a distorted Y-shaped structure containing bundles of three helices connected by short loops (Fig. 2 ▶). Two views of the CarD83–161 electrostatic surface (180° apart) showing the distribution of positive (blue) and negative (red) surface charge are shown in Fig. 2 ▶. The protein carries a pronounced overall positive charge at the N-terminus (helices H1 and H2) and a negative charge in the H3 helix. In the CarD83–161 structure, the first helix (H1) is connected to the second helix (H2) by a three-residue loop. The second helix (H2) is connected to the third helix (H3) by an eight-residue loop. The lengths of the various helices were observed to be 21 Å for the H1 helix, 24 Å for the H2 helix and 60 Å for the H3 helix. The following interactions were observed between the H1, H2 and H3 helices: (i) a salt bridge Lys95 Nζ⋯Glu106 O∊1 between the H1 and H2 helices, (ii) salt bridges Trp85 N∊1⋯Glu124 O∊2 and Arg88 NH2⋯Glu124 O∊2 between the H1 and H3 helices and (iii) a salt bridge Asp115 Oδ1⋯Lys125 Nζ between the H2 and H3 helices. These residues are highly conserved in the sequences of CarD from all mycobacterial species. These results indicate that the CarD83–161 structure is unique and is a template for the structure of CarD from all mycobacterial species.
Figure 2.
Crystal structure of the C-terminal domain of MtbCarD (CarD83–161). (a) CarD83–161 adopts a distorted Y-shaped structure. Two views of a ribbon diagram of the CarD83–161 crystal structure are shown (180° apart). (b) Two views of the molecular surface of CarD83–161 coloured by electrostatic potential (180° apart). The surface potential ranges from −10kT (red denotes negative charge) to +10kT (blue denotes positive charge).
PISA server (Krissinel & Henrick, 2007 ▶) analysis of the CarD83–161 interfaces indicated that the quaternary structure of CarD83–161 has two macromolecular oligomeric states with a buried surface area of 1837.1 Å2, a solvent-accessible surface area of 100 049.5 Å2 and a dissociation barrier of 5.2 kcal mol−1. Of the 79 residues of CarD83–161, 76 residues constitute the total surface area of 5943.3 Å2 with a free energy (ΔG) of −48.9 kcal mol−1. The server identified four interfaces in the CarD83–161 structure, with surface areas of 918.6 Å2 for surface 1, 364.8 Å2 for surface 2, 250.7 Å2 for surface 3 and 98.0 Å2 for surface 4.
3.4. Identification of the CarD83–161 fold
Analysis of the CarD83–161 sequence using the leucine-zipper domain server (Bornberg-Bauer et al., 1998 ▶) indicated that CarD83–161 does not contain a leucine-zipper domain as reported previously (Stallings et al., 2009 ▶). The trimeric helix bundle formed by the CarD83–161 structure did not superpose on any leucine-zipper domain structure available in the database. The DALI server (Holm & Rosenström, 2010 ▶) was used to obtain the homologous structure of CarD83–161, which indicated a unique fold with no precedents.
A previous study (Westblade et al., 2010 ▶) indicated that MtbCarD is like a CdnL protein, which shares similarity to the N-terminal TRCF-RID-like domain and lacks an identifiable C-terminal DNA-binding domain. When the CarD83–161 structure was aligned with the following HMG domain-containing proteins involved in DNA binding, (i) the three-dimensional structure of the human SRY-DNA complex solved by multidimensional heteronuclear-edited and filtered NMR (PDB entry 1hry; Werner et al., 1995 ▶), (ii) the HMG domain structure (from mouse) complexed with DNA from NMR (PDB entry 2lef; Love et al., 1995 ▶) and (iii) the NMR structure of rat HMG1 HMGA fragment (PDB entry 1aab; Hardman et al., 1995 ▶), no sequence or structure homology was observed between CarD83–161 and these proteins. These results indicate that CarD83–161 contains a unique fold that was not observed in the existing structural database.
3.5. Comparison of the CarD83–161 structure with M. tuberculosis and T. thermophilus full-length CarD structures
Recently, the crystal structures of full-length M. tuberculosis CarD in complex with RNAP β lobes (Gulten & Sacchettini, 2013 ▶; PDB entry 4kbm) and the crystal structure of T. thermophilus CarD (TthCarD; Srivastava et al., 2013 ▶; PDB entry 4l5g) have been determined. The structure of the C-terminal domain of MtbCarD and TthCarD consists of five α-helices, compared with the three α-helices observed in our MtbCarD83–161 structure. Ala122–Ala160 of our MtbCarD83–161 structure form a single α3 helix, while Ala122–Ala159 of full-length MtbCarD and TthCarD form a helix–turn–helix motif (Gulten & Sacchettini, 2013 ▶; Srivastava et al., 2013 ▶; Fig. 3 ▶). In the MtbCarD and TthCarD structures, Ala122–Glu143 form helix α5 and Lys149–Ala159 form helix α6 and the helices are connected by a five-residue loop. Superposition of the MtbCarD83–161 structure with the TthCarD structure indicated an r.m.s.d of 1.37 Å for 58 Cα atoms, while superposition with MtbCarD gave an r.m.s.d. of 0.89 Å for 59 Cα atoms (Fig. 3 ▶).
Figure 3.
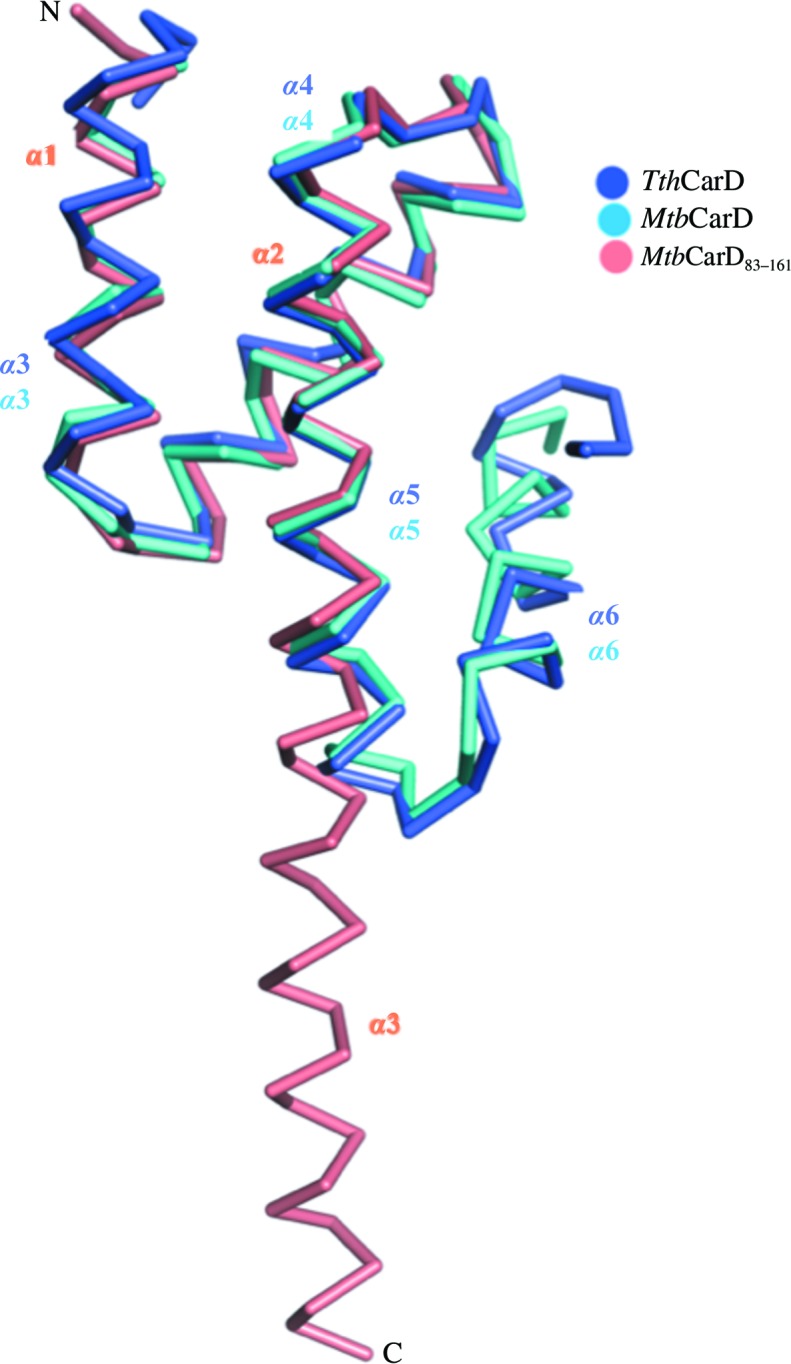
Superposition of MtbCarD83–161 with the crystal structures of the C-terminal domains of full-length MtbCarD (Gulten & Sacchettini, 2013 ▶; PDB entry 4kbm) and TthCarD (Srivastava et al., 2013 ▶; PDB entry 4l5g). MtbCarD83–161 is shown in orange and is labelled as described in the text. The C-terminal domains of full-length MtbCarD andTthCarD are shown in cyan and blue, respectively, and all helices are labelled according to the corresponding publications.
The α5 helix of the C-terminal domain of MtbCarD and TthCarD superposes well with the α3 helix of MtbCarD83–161. However, a segment containing a loop and helix α6 (residues 143–161) of MtbCarD and TthCarD is oriented ∼180° away from the α3 helix of MtbCarD83–161. In the full-length MtbCarD and TthCarD structures (Gulten & Sacchettini, 2013 ▶; Srivastava et al., 2013 ▶), residues 71–75 of helix 1 interact with residues 153–157 of the C-terminal domain of CarD in a mostly hydrophobic manner. N-terminal sequencing of our crystallized protein indicated that residues 76–161 of CarD are present in our crystals. This means that the hydrophobic interactions between residues 71–75 and residues 153–157 are missing in our MtbCarD83–161 structure and this has caused the 143–161 helix of our structure to unfold. It indicates that the structural differences in our MtbCarD83–161 structure versus full-length M. tuberculosis and T. thermophilus CarD are owing to a combination of proteolysis and crystallization artifacts.
In the current study, we have expressed and purified full-length MtbCarD and TaqRNAP β subunit. The MtbCarD protein eluted as a monomer, while the TaqRNAP β subunit eluted as an aggregated protein from a gel-filtration column. We have determined the structure of the C-terminal domain of MtbCarD (CarD83–161). Comparison of our MtbCarD83–161 structure with recently determined full-length M. tuberculosis and T. thermophilus CarD crystal structures has revealed structural differences in the C-terminal region of our MtbCarD83–161 structure owing to proteolysis and crystallization artifacts.
Supplementary Material
PDB reference: CarD83–161, 4kmc
Supplementary Fig. S1. Circular-dichroism analysis of full-length MtbCarD. (A) CD spectra of MtbCarD were recorded from 260 to 200 nm at 250C and normalized to the mean residue ellipticity. The secondary-structure content of MtbCarD was quite similar to the secondary-structure content obtained from theoretical prediction analysis. (B) PSIPRED analysis of full-length MtbCarD.. DOI: 10.1107/S2053230X13034407/gx5219sup1.tif
Supplementary Table S1. N-terminal sequencing of CarD protein obtained from dissolved crystals used for the X-ray diffraction experiment.. DOI: 10.1107/S2053230X13034407/gx5219sup2.pdf
Acknowledgments
This work was supported by a research grant from the Council of Scientific and Industrial Research (CSIR), New Delhi, India. Grants from UGC Networking, Capacity Buildup and a Department of Science and Technology (DST) purse grant are gratefully acknowledged. The authors declare that they have no competing interests. The authors thank Dr Sushma Nagpal for allowing them to use the BIAcore facility of the National Institutes of Immunology, New Delhi for the SPR experiment. The authors thank the staff of the BM14 beamline for their help in CarD intensity data collection and processing. We acknowledge the support of Dr Seth A. Darst from Rockefeller University, New York, USA in gifting us the plasmid containing the TaqRNAP β subunit gene.
Footnotes
Supporting information has been deposited in the IUCr electronic archive (Reference: GX5219).
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. (2011). Acta Cryst. D67, 271–281. [DOI] [PMC free article] [PubMed]
- Bornberg-Bauer, E., Rivals, E. & Vingron, M. (1998). Nucleic Acids Res. 26, 2740–2746. [DOI] [PMC free article] [PubMed]
- Bradford, M. M. (1976). Anal. Biochem. 72, 248–254. [DOI] [PubMed]
- DeLano, W. L. (2002). PyMOL. http://www.pymol.org.
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Gulten, G. & Sacchettini, J. C. (2013). Structure, 21, 1859–1869. [DOI] [PMC free article] [PubMed]
- Hardman, C. H., Broadhurst, R. W., Raine, A. R., Grasser, K. D., Thomas, J. O. & Laue, E. D. (1995). Biochemistry, 34, 16596–16607. [DOI] [PubMed]
- Holm, L. & Rosenström, P. (2010). Nucleic Acids Res. 38, W545–W549. [DOI] [PMC free article] [PubMed]
- Jones, D. T. (1999). J. Mol. Biol. 292, 195–202. [DOI] [PubMed]
- Krissinel, E. & Henrick, K. (2007). J. Mol. Biol. 372, 774–797. [DOI] [PubMed]
- Love, J. J., Li, X., Case, D. A., Giese, K., Grosschedl, R. & Wright, P. E. (1995). Nature (London), 376, 791–795. [DOI] [PubMed]
- Pape, T. & Schneider, T. R. (2004). J. Appl. Cryst. 37, 843–844.
- Srivastava, D. B., Leon, K., Osmundson, J., Garner, A. L., Weiss, L. A., Westblade, L. F., Glickman, M. S., Landick, R., Darst, S. A., Stallings, C. L. & Campbell, E. A. (2013). Proc. Natl Acad. Sci. USA, 110, 12619–12624. [DOI] [PMC free article] [PubMed]
- Stallings, C. L., Stephanou, N. C., Chu, L., Hochschild, A., Nickels, B. E. & Glickman, M. S. (2009). Cell, 138, 146–159. [DOI] [PMC free article] [PubMed]
- Weiss, L. A., Harrison, P. G., Nickels, B. E., Glickman, M. S., Campbell, E. A., Darst, S. A. & Stallings, C. L. (2012). J. Bacteriol. 194, 5621–5631. [DOI] [PMC free article] [PubMed]
- Werner, M. H., Huth, J. R., Gronenborn, A. M. & Clore, G. M. (1995). Cell, 81, 705–714. [DOI] [PubMed]
- Westblade, L. F., Campbell, E. A., Pukhrambam, C., Padovan, J. C., Nickels, B. E., Lamour, V. & Darst, S. A. (2010). Nucleic Acids Res. 38, 8357–8369. [DOI] [PMC free article] [PubMed]
- Whitmore, L. & Wallace, B. A. (2004). Nucleic Acids Res. 32, W668–W673. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- World Health Organization (2011). Global Tuberculosis Report Geneva: World Health Organization. http://www.who.int/tb/publications/global_report/en/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: CarD83–161, 4kmc
Supplementary Fig. S1. Circular-dichroism analysis of full-length MtbCarD. (A) CD spectra of MtbCarD were recorded from 260 to 200 nm at 250C and normalized to the mean residue ellipticity. The secondary-structure content of MtbCarD was quite similar to the secondary-structure content obtained from theoretical prediction analysis. (B) PSIPRED analysis of full-length MtbCarD.. DOI: 10.1107/S2053230X13034407/gx5219sup1.tif
Supplementary Table S1. N-terminal sequencing of CarD protein obtained from dissolved crystals used for the X-ray diffraction experiment.. DOI: 10.1107/S2053230X13034407/gx5219sup2.pdf