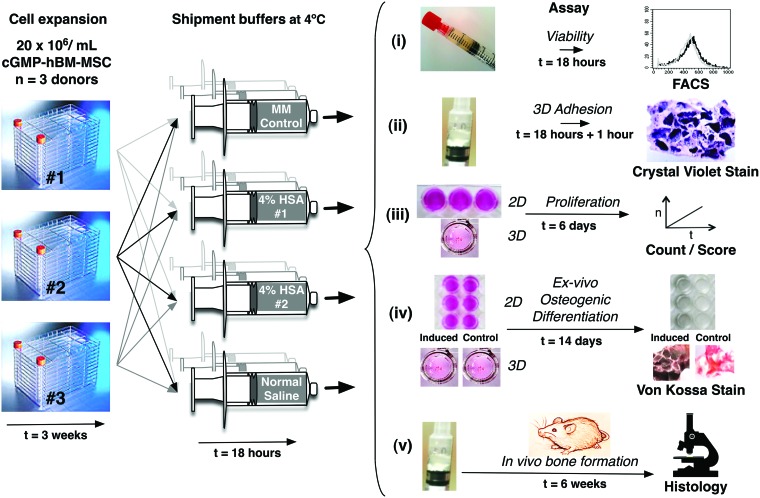
FIG. 1.
Overview of experiments. hBM-MSC harvested from three independent donors at the cGMP facilities were harvested and maintained for 18 h at 4°C in syringes according to the MM control and three transportation conditions shown. After 18 h, the cells from each syringe were analyzed to assess cell quality with regard to (i) viability; (ii) adhesion to a 3D scaffold; (iii) proliferation potential of cells kept as 2D monolayers or attached to 3D scaffold; (iv) ex vivo osteogenic differentiation potential of cells kept as 2D monolayers or cells attached to 3D scaffold; and (v) in vivo bone formation of cGMP-BM-MSC/HA/β-TCP xenografts analyzed after 6 weeks by histology. hBM-MSC, human bone marrow-derived mesenchymal stromal/stem cells; cGMP, current Good Manufacturing Practice; HA/β-TCP, 20% hydroxyapatite and 80% β-tri-calcium phosphate; MM, maintenance medium. Color images available online at www.liebertpub.com/tec