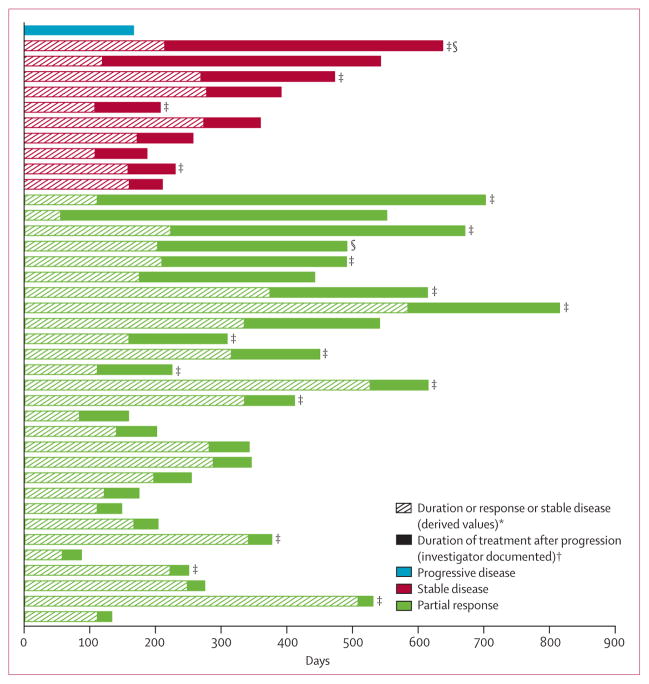
Figure 3. Duration of initial response or stable disease and of ongoing crizotinib treatment in patients who continued to receive crizotinib after progression.
Patients are ordered by initial best response before progression and duration of crizotinib treatment after progression (n=39). *Defined as the time (in weeks) from the first documentation of objective tumour response (complete response or partial response) that was subsequently confirmed, to the first documentation of progressive disease or death. Stable disease duration was calculated from the date of the first dose to the date of first documented disease progression. †Defined as time from investigator-documented progressive disease to the last date of crizotinib dose or censor at the time of analysis. Disease progression and best objective response were derived according to Response Evaluation Criteria in Solid Tumors. ‡Treatment ongoing at the time of analysis. §Received crizotinib as first-line treatment.