Abstract
Purpose
To assess whether antidepressant prescribing during pregnancy decreased following release of U.S. and Canadian public health advisory warnings about the risk of perinatal complications with antidepressants.
Methods
We analyzed data from 228,876 singleton pregnancies among women (aged 15–44 years) continuously enrolled in Tennessee Medicaid with full pharmacy benefits (1995–2007). Antidepressant prescribing was determined through outpatient pharmacy dispensing files. Information on sociodemographic and clinical factors was obtained from enrollment files and linked birth certificates. An interrupted time-series design with segmented regression analysis was used to quantify the impact of the advisory warnings (2002–2005).
Results
Antidepressant prescribing rates increased steadily from 1995–2001, followed by sharper increases from 2002–late 2004. Overall antidepressant prescribing prevalence was 34.51 prescriptions (95% CI 33.37–35.65) per 1,000 women in January 2002, and increased at a rate of 0.46 (95% CI 0.41–0.52) prescriptions per 1,000 women per month until the end of the pre-warning period (May 2004). During the post-warning period (October 2004 – June 2005), antidepressant prescribing decreased by 1.48 (95% CI 1.62-1.35) prescriptions per 1,000 women per month. These trends were observed for both SSRI and non-SSRI antidepressants, although SSRI prescribing decreased at a greater rate.
Conclusion
Antidepressant prescribing to pregnant women in Tennessee Medicaid increased from 1995–late 2004. U.S. and Canadian public health advisories about antidepressant-associated perinatal complications were associated with steady decreases in antidepressant prescribing from late 2004 until the end of the study period, suggesting that the advisory warnings were impactful on antidepressant prescribing in pregnancy.
Keywords: antidepressants, pregnancy, pregnant women, selective serotonin reuptake inhibitors, trends, practice patterns, regulatory warnings
Introduction
Antidepressants are widely prescribed for major depression and other psychiatric disorders that commonly occur in women of reproductive age (Andrade et al. 2008; Ramos et al. 2007; Ververs et al. 2006). They are considered the primary treatment, or important adjuncts, for moderate to severe depression (Bauer et al. 2002; Davidson 2010; Lam et al. 2009) and other indications (Canadian Psychiatric Association 2006; Isper and Stein 2012; Kroenke et al. 2009), although their effectiveness and safety in the context of pregnancy have been seldom studied (Yonkers et al. 2009). In pregnant women with depression, antidepressants have been shown to reduce depressive symptoms and improve maternal functioning (Wisner et al. 2009a), while antidepressant discontinuation has been associated with increased risk of antenatal depressive relapses in some (Cohen et al. 2006) but not all studies (Yonkers et al., 2011).
For several years, selective serotonin reuptake inhibitors (SSRIs), the most commonly prescribed antidepressants (Olfson and Marcus 2009), were regarded as safe for use in pregnancy (Koren and Nordeng 2012). This perception may have changed beginning in late 2004 following the release of public health advisory warnings about the risk of perinatal complications with SSRIs and other antidepressants by the U.S. Food and Drug Administration (FDA) in June 2004 (US Food and Drug Authority 2004), and by Health Canada two months later (Health Canada 2004). These warnings were prompted by increasing reports of adverse neonatal outcomes associated with maternal antidepressant use including potential risk for cardiovascular malformations (Wurst et al. 2010). Results of antidepressant reproductive safety studies, however, have been inconclusive or conflicting (Einarson and Einarson 2005; Hemels et al. 2005; Koren and Nordeng 2012), and many adverse neonatal outcomes associated with fetal antidepressant exposure have also been linked with untreated gestational depression (Bonari et al. 2004; Wisner et al. 2009), causing uncertainty about the risks versus benefits of antidepressant use in pregnancy (Kuehn 2009).
Neither regulatory warning advised against the use of antidepressants or recommended antidepressant discontinuation during pregnancy, but they received wide media coverage (Einarson et al. 2005), and could have made practitioners more hesitant to prescribe antidepressants to pregnant women (Bilszta et al. 2011). However, the impact of these regulatory actions on antidepressant prescribing in pregnant women is relatively unknown. To address this knowledge gap, we utilized data from a recently completed cohort study of antidepressants and the risk of adverse neonatal outcomes (Hayes et al. 2012) to assess whether antidepressant prescribing decreased among pregnant women after the release of the U.S. and Canadian advisory warnings in late 2004.
Methods
Setting and population
This study utilized data from a recent cohort study of antidepressants and the risk of adverse neonatal outcomes using data from Tennessee Medicaid (Hayes et al 2012), an expanded version of the joint federal-state Medicaid program that finances medical care for qualifying low income persons. The computerized Medicaid files included an enrollment file, as well as files recording prescriptions filled at pharmacies, hospital admissions, outpatient visits, and long-term care residence. Medicaid files were linked with birth certificate data, which included information on sociodemographic (maternal age, race/ethnicity, education), medical (parity, maternal smoking status) and reproductive factors (infant sex and birth weight, date of maternal last menstrual period [LMP]).
Linkages between these data sources permitted the identification of 228,876 singleton pregnancies among women enrolled in the Tennessee Medicaid program from 1995–2007. Eligible women were 15–44 years of age on the date of delivery, and had 180 days of continuous Medicaid enrollment, with pharmacy benefits, prior to their LMP through 90 days after delivery. Brief administrative gaps in enrollment of 45 days or less were allowed.
The first day of pregnancy was defined as the date of the maternal LMP. The LMP listed on the birth certificate was used to define the start of pregnancy and to estimate gestational dates for 85.3% of cohort members. When the LMP was not available from birth certificates, the LMP was set to the median gestational age for infants of the same birth year, birthweight and race, or was assigned based on a gestational period of 273 days.
Approval for this study was granted by the Institutional Review Boards of Vanderbilt University and the Tennessee Department of Health.
Measures
The primary outcome was maternal filling of antidepressant prescriptions, determined from Medicaid pharmacy files, which we used to estimate antidepressant prescribing. Pharmacy files included the medication name, prescription fill date, quantity dispensed, and number of days for which the medication was supplied. The days of supply and quantity dispensed were used to estimate the days of exposure represented by a prescription. Filled prescriptions of antidepressant medications (Supplemental Table 1), which allow a maximum of 30 days of supply, were counted for the 180 days prior to LMP through the date of delivery, and were classified as SSRIs, serotonin-norepinephrine re-uptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), or other antidepressants, based on their pharmacological activity (Lanni et al., 2009).
Antidepressant prescribing during pregnancy was defined as the filling of any antidepressant prescription between the LMP and date of delivery. Two or more antidepressant prescriptions occurring on the same date were counted as a single antidepressant prescription if the drugs were identical, or were from the same class. For example, two prescriptions for fluoxetine or two different SSRIs occurring on the same date were counted as a single prescription. Prescriptions that occurred on different dates, or occurred on the same date but involved agents from differing drug classes, were counted separately.
Maternal diagnoses were identified in electronic records of medical care encounters using International Classification of Diseases, 9th Revision (ICD-9) diagnosis codes associated with any inpatient or outpatient claim during the time window beginning 180 days prior to LMP and ending on the LMP date. These included diagnosis codes consistent with a unipolar depressive disorder (ICD-9 296.2, 296.3, 300.4, or 311), a bipolar spectrum disorder, any anxiety or substance use disorder, and common or severe maternal medical conditions. The latter included asthma, chronic obstructive pulmonary disease, chronic cardiac disease, diabetes mellitus, immunodeficiency, renal disease, and malignancy.
Statistical analysis
Maternal clinical and demographic characteristics were presented as number (percentage) and median (inter-quartile range), as appropriate. To examine prescribing trends over the entire study period (January 1, 1995 – December 31, 2006), we calculated the rate of antidepressant prescribing for each birth year, defined as the number of antidepressant prescriptions per 1,000 pregnant women.
We used interrupted time-series analysis to examine the association between release of the U.S. and Canadian Health Advisories and antidepressant prescribing. In August 2005, the Tennessee Medicaid program set a limit on the number of covered prescription drugs. Thus, for the interrupted time-series analysis, study time was restricted to January 1, 2002 through July 31, 2005, and was divided into three periods. The period before the FDA pregnancy warning was issued (pre-warning period) included the 29 months from January 1, 2002 to May 31, 2004. A transition period was defined as the 4 months (June 1, 2004 to September 30, 2004) encompassing the regulatory warnings from the FDA, which were the most pertinent to the analysis, and from Health Canada, which also received extensive coverage in the media and could have influenced prescribing of antidepressants to pregnant women in the U.S. The post-warning period included the 10 months from October 1, 2004 to July 31, 2005.
We calculated antidepressant prescribing rates for each study month, defined as the total number of prescriptions during that month per 1,000 pregnant women. This unit of measure was chosen because cohort members could fill an antidepressant prescription at any time between their estimated LMP date and date of delivery, and multiple dispensings from a single prescription were unlikely given that Tennessee Medicaid allowed a maximum of 30 days of supply for a prescription fill. Prescriptions for women who contributed at least 15 days of her pregnancy time in a particular month were counted for that month. Segmented linear regression models were used to estimate antidepressant prescribing rates and 95% confidence intervals (CIs), and compare linear trends in the prevalence of antidepressant prescribing during pre- and post-warning periods. The regression models estimated the rate of antidepressant prescribing at the beginning of the pre- and post-warning segments (intercept) and rates of change in antidepressant prescribing (slope) in each segment. Changes in intercept for the post-warning segment indicated rapid emergence of an association between the regulatory pregnancy warnings and rates of antidepressant prescribing. Changes in slope during the post-warning segment, as compared with the pre-warning segment, indicated a change in antidepressant prescribing trend (accounting for both the direction and rate of change) associated with regulatory pregnancy warning issuance. The regression models included a term for the effect of the FDA pregnancy warning, and adjusted for linear trends in antidepressant prescribing during the pre- and post-warning periods. We corrected the models for the serially autocorrelated nature of the observations (Shadish et al. 2002)—that is, the tendency for prescribing patterns at closer time points to be more similar than those occurring further apart that, if uncorrected, can lead to overestimation of intervention effects (Wagner et al. 2002). We also presented the monthly antidepressant prescribing rates graphically. Smoothed lines were drawn over time using a locally-weighted polynomial regression to smooth the data (Becker et al., 1988; Cleveland, 1979, 1981).
In late December 2003 and October 2004, the Committee on Safety of Medicines (CSM) in the United Kingdom (Committee on Safety of Medicines’ Expert Working Group, 2003) and the U.S. FDA (Food and Drug Administration, 2004) issued additional public health advisories warning about possible SSRI-associated increased risk of suicidal behavior in young people (Supplemental Figure 1). Although these warnings did not address any issues related to pregnancy, they also received extensive media coverage and could have influenced antidepressant use in our cohort (Huybrechts et al. 2013). Thus, a sensitivity analysis was conducted in order to model the effect of both regulatory warnings (regarding perinatal risks and suicidal behavior) using an expanded transition period (January 1, 2004 to October 31, 2004). Additional analyses stratified by age were also conducted to examine prescribing changes in non-pediatric age groups (e.g., patients aged 18–24 years and those aged 25 years and older) that were not targeted by the suicide warnings. The upper bound of the 18–24 year old stratum corresponds to the World Health Organization definition of youth (World Health Organization, 1989).
All analyses were performed using SAS (version 9.1; SAS Institute Inc., Cary, NC) and R (version 2.11.1; www.r-project.org) statistical software. A two-sided 5% significance level was used for all statistical inferences.
Results
Cohort Demography and Clinical Characteristics
The majority of cohort members were young, Caucasian or African-American, unmarried, urban- or suburban-dwelling women who had a high school or lower level of education (Table 1). Approximately 6% (n = 16,896) of cohort members filled at least one prescription for an antidepressant during pregnancy in the study period (1995–2007), and were considered antidepressant users. Antidepressant users tended to be predominantly Caucasian, with higher proportions of married persons, rural residence, smoking during pregnancy, substance abuse diagnoses, and general medical comorbidity. The predominant maternal psychiatric diagnoses among antidepressant users were unipolar depressive and anxiety disorders, while nearly 7% of antidepressant users had a bipolar spectrum disorder diagnosis (Bipolar I or II Disorder; or Bipolar Disorder, Not Otherwise Specified).
Table 1.
Maternal clinical and demographic characteristics for entire cohort and women who were prescribed antidepressants before and after FDA and Health Canada advisories
| Entire cohort | Prescribed antidepressants | |||||
|---|---|---|---|---|---|---|
| 1995–2007 | 1995–2007 | 2002–2005 | ||||
| N | 228,876 | 16,896 | 8,561 | |||
| Median age (inter-quartile range), years | 22 (19–26) | 24 (21–29) | 24 (21–28) | |||
| N | % | N | % | N | % | |
| Race, no. (%) | ||||||
| Caucasian | 127,592 | 55.7 | 13,935 | 82.5 | 7,102 | 83.0 |
| African-American | 95,503 | 41.7 | 2,523 | 14.9 | 1,252 | 14.6 |
| Other | 5,781 | 2.6 | 438 | 2.6 | 207 | 2.4 |
| Married, no. (%) | ||||||
| Married | 74,805 | 32.7 | 7,178 | 42.5 | 3,649 | 42.6 |
| Unmarried | 153,970 | 67.3 | 9,704 | 57.4 | 4,902 | 57.3 |
| Unknown | 101 | <0.1 | 14 | 0.1 | 10 | 0.1 |
| Residence, no. (%), n = 228,395 | ||||||
| Urban | 113,890 | 49.8 | 6,139 | 36.3 | 3,192 | 37.3 |
| Suburban | 51,316 | 22.4 | 4,848 | 28.7 | 2,439 | 28.5 |
| Rural | 63,189 | 27.6 | 5,874 | 34.8 | 2,911 | 34.0 |
| Unknown | 481 | 0.2 | 35 | 0.2 | 19 | 0.2 |
| Education, no. (%) | ||||||
| Less than high school | 96,170 | 42.0 | 6,335 | 37.5 | 3,095 | 36.2 |
| High school | 98,224 | 42.9 | 7,331 | 43.4 | 3,808 | 44.5 |
| Greater than high school | 33,956 | 14.8 | 3,185 | 18.9 | 1,627 | 19.0 |
| Unknown | 526 | 0.3 | 45 | 0.2 | 31 | 0.3 |
| Parity | ||||||
| Primiparous | 67,265 | 29.4 | 4,082 | 24.2 | 2,087 | 24.4 |
| 1 | 79,742 | 34.8 | 5,815 | 34.4 | 2,910 | 34.0 |
| 2 | 45,813 | 20.0 | 3,934 | 23.3 | 2,011 | 23.5 |
| 3+ | 35,545 | 15.5 | 3,023 | 17.9 | 1,529 | 17.9 |
| Unknown | 511 | 0.3 | 42 | 0.2 | 24 | 0.2 |
| Diagnosed depression before pregnancy, no. (%) | 13,593 | 4.7 | 5,196 | 30.8 | 2,763 | 32.2 |
| Diagnosed anxiety disorder, no. (%) | 17,958 | 7.8 | 5,997 | 35.5 | 3,292 | 38.5 |
| Diagnosed bipolar disorder, no. (%) | 4,897 | 1.7 | 1,115 | 6.6 | 625 | 7.3 |
| General medical comorbidity, no. (%)b | 21,232 | 9.3 | 3,175 | 18.8 | 1,723 | 20.1 |
| Smoking in pregnancy, no. (%) | ||||||
| No | 160,242 | 70.0 | 8,671 | 51.3 | 4,359 | 50.9 |
| Yes | 68,248 | 29.8 | 8,207 | 48.6 | 4,198 | 49.0 |
| Unknown | 386 | 0.2 | 18 | 0.1 | 4 | 0.1 |
| Diagnosed substance abuse, no. (%) | 34,353 | 15.0 | 5,827 | 34.5 | 3,502 | 40.9 |
| Antidepressant prescriptions filled during pregnancy, no. (%) | ||||||
| None | 211,980 | 92.6 | … | … | … | … |
| 1–2 | 10,700 | 4.7 | 10,700 | 63.3 | 5,230 | 61.1 |
| 3+ | 6,196 | 2.7 | 6,196 | 36.7 | 3,331 | 38.9 |
Pre-warning period included women who delivered before June 2004, while post-warning period included women who delivered after September 2004; 5.834 women who delivered between June and September 2004 were excluded.
Includes diagnosis of asthma, chronic cardiac disease, diabetes mellitus, immunodeficiency, renal disease, mental retardation, and chronic obstructive pulmonary disease.
Characteristics of the antidepressant users (n = 8,561) for the interrupted time-series analysis (2002–2005) did not differ significantly from antidepressant users from the entire study period (1995–2007) (Table 1). As such, the restricted antidepressant user cohort for time-series analysis was considered representative of the broader group of antidepressant users.
Antidepressant Prescribing, 1995–2007
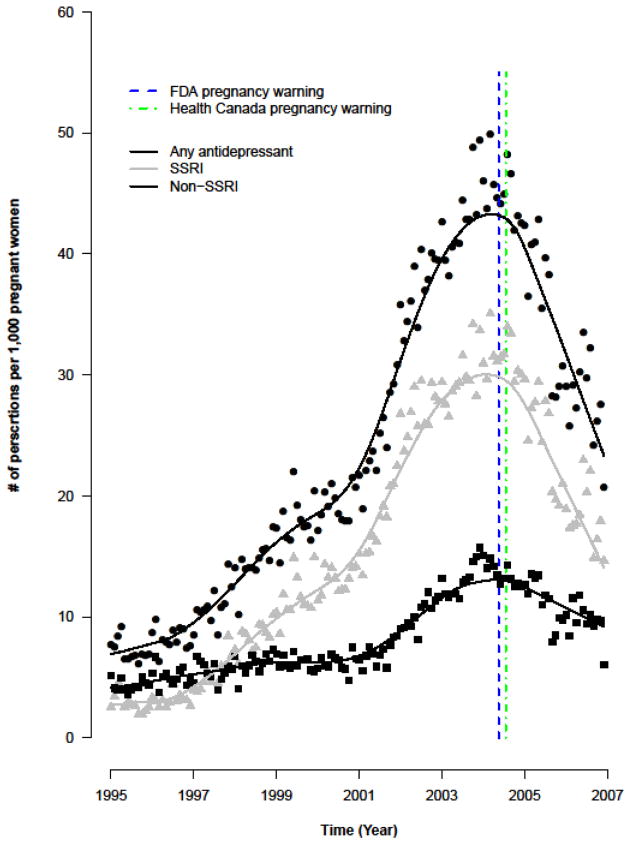
Prescribing rates for antidepressants in general, and SSRIs in particular, increased steadily between 1995 and 2001, followed by sharper increases between 2002 and late 2004 (Figure 1). From 2004 onward, a reversal of this trend was observed (Table 2), the onset of which occurred in very close proximity to issuance of the FDA pregnancy warning. These general trends were observed for SSRIs and most non-SSRI antidepressants (Table 2), although the magnitude observed changes were most pronounced for SSRIs. TCA prescribing initially eclipsed SSRI prescribing in 1995, but steadily decreased throughout the study period (Table 2).
Figure 1.
Antidepressant prescribing rates during pregnancy (1995–2007). The dates of FDA (blue dashed line) and Health Canada (green dashed line) pregnancy warnings are also shown. Smoothed lines were drawn for antidepressant prescribing rates over time using a locally-weighted polynomial regression to smooth the data.
Table 2.
Rates of antidepressant prescribing during pregnancy (1995–2007), by drug class
| Year | Number of | Rates of Antidepressant Prescribing | ||||
|---|---|---|---|---|---|---|
| women | (Number of prescriptions per 1,000 women)a | |||||
| (N) | Any | Other | ||||
| Antidepressant | SSRI | SNRI | TCA | Antidepressant | ||
| 1995 | 14,844 | 66.0 | 27.1 | 0.5 | 32.3 | 8.8 |
| 1996 | 16,248 | 64.9 | 24.9 | 0.8 | 32.7 | 8.9 |
| 1997 | 16,071 | 86.3 | 38.6 | 1.8 | 37.0 | 13.3 |
| 1998 | 17,226 | 115.9 | 70.4 | 4.4 | 36.2 | 14.5 |
| 1999 | 18,018 | 147.1 | 95.8 | 7.6 | 37.5 | 18.2 |
| 2000 | 18,788 | 162.0 | 115.1 | 12.3 | 29.0 | 15.3 |
| 2001 | 19,155 | 182.8 | 135.9 | 20.8 | 20.3 | 21.5 |
| 2002 | 19,687 | 290.8 | 224.8 | 39.0 | 20.3 | 26.1 |
| 2003 | 17,951 | 358.8 | 266.8 | 66.4 | 22.0 | 29.2 |
| 2004 | 17,606 | 397.6 | 289.1 | 83.4 | 23.0 | 28.8 |
| 2005 | 18,198 | 359.8 | 259.6 | 77.4 | 18.7 | 24.6 |
| 2006 | 19,132 | 267.4 | 184.0 | 58.2 | 16.8 | 15.5 |
| 2007 | 15,952 | 195.4 | 128.1 | 43.3 | 14.4 | 16.3 |
Represents the total number of prescriptions during pregnancy per 1,000 women who gave birth in a given study year.
Effect of the U.S. FDA Pregnancy Warning on Antidepressant Prescribing, 2002–2005
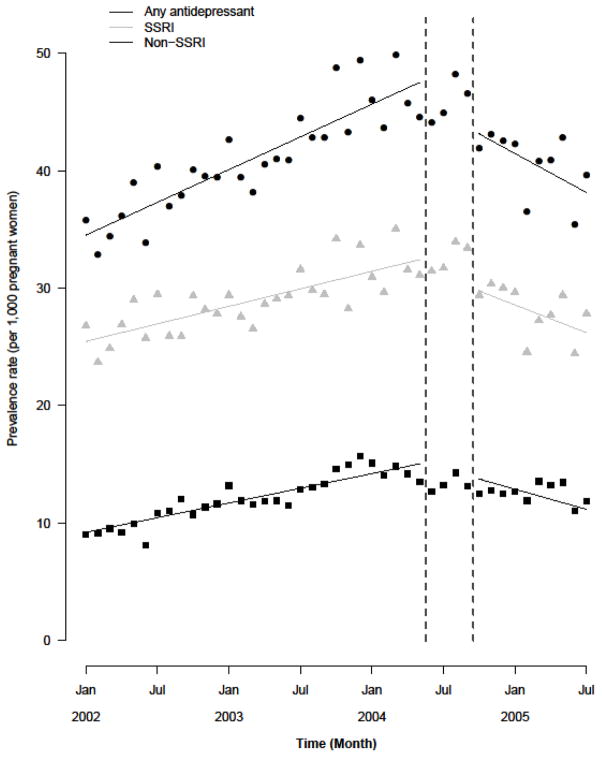
Antidepressant prescribing increased steadily during the pre-warning period, followed by a sharp decrease in the post-warning period (Figure 2A). Overall antidepressant prescribing prevalence was 34.51 prescriptions (95% CI 33.37–35.65) per 1,000 women at the beginning of the pre-warning period, and increased at a rate of 0.46 prescriptions per 1,000 women per month until the end of the pre-warning period (Table 3). The FDA pregnancy warning was associated with a significant slope change (−1.02, 95% CI −1.5, −0.5) representing a change in prescribing trend. During the post-warning period, overall antidepressant prescribing rate decreased by 1.48 prescriptions per 1,000 women per month. These trends were observed for both SSRI and non-SSRI antidepressants, although SSRI prescribing decreased at a greater rate than non-SSRI prescribing (Table 3).
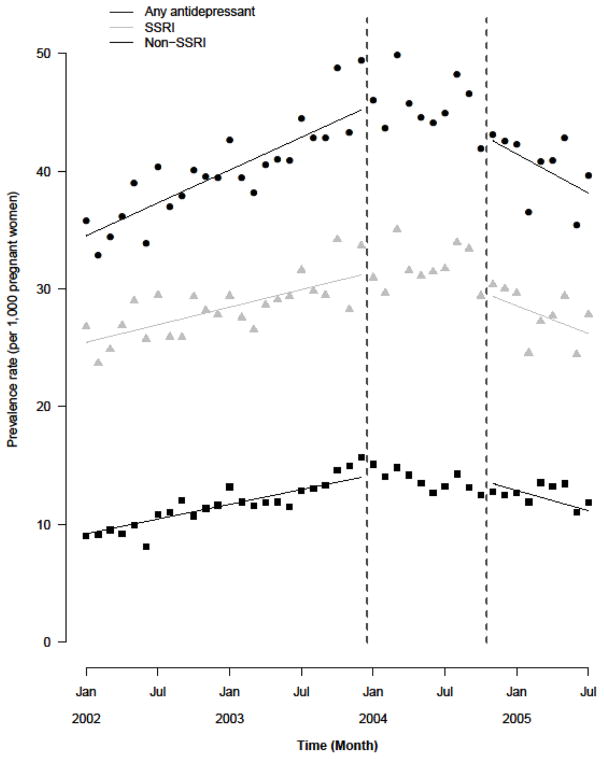
Figure 2.
Figure 2A. Results of the interrupted time-series analysis focused on the association between release of the FDA and Health Canada pregnancy warnings and antidepressant prescribing during pregnancy, using a 4 month transition period (June 1, 2004–September 30, 2004).
Figure 2B. Results of the interrupted time-series analysis that estimated the association between pregnancy and suicide warnings and antidepressant prescribing during pregnancy, using an expanded transition period (January 1, 2004–October 31, 2004).
Table 3.
Relationship between release of U.S. FDA pregnancy warning and rates and trends of antidepressant prescribing (per 1,000 persons) during pregnancy, 2002–2005a
| Pre-Warning Period (January 1, 2002 – May 31, 2004) | Post-Warning Period (October 1, 2004 – July 31, 2005) | |||
|---|---|---|---|---|
| Antidepressant prescribing at the start of observation period, N prescriptions per 1,000 womenb | Rate of change in antidepressant use, N prescriptions per 1,000 women/month (95% CI)c | Magnitude of change in October 2004, N prescriptions per 1,000 women (95% CI)d | Rate of change in antidepressant use, N prescriptions per 1,000 women/month (95% CI)e | |
| Any antidepressant | 34.51 | 0.46 (0.41, 0.52) | −3.81 (−7.17, −0.44) | −1.48 (−1.62, −1.35) |
| SSRI | 25.46 | 0.25 (0.20, 0.30) | −2.26 (−4.27, −0.25) | −0.89 (−0.93, −0.85) |
| Non-SSRI | 9.19 | 0.21 (0.19, 0.23) | −1.00 (−2.12, 0.13) | −0.70 (−0.73, −0.68) |
Change associated with FDA pregnancy warning represents the estimated effect of the U.S. FDA pregnancy warning (issued June 9, 2004), using a 4 month transition period (June 1, 2004 – September 30, 2004) that also encompassed Health Canada pregnancy advisory.
Represents the estimated number of antidepressant prescriptions per 1,000 pregnant women in January 2004.
Represents the rate of change (slope) in antidepressant prescribing during the pre-warning period. The positive values (with 95% confidence intervals [CIs]) are rates of increase, expressed as number of prescriptions per 1,000 pregnant women per month.
Represents the difference between the estimated antidepressant prescribing rate (number of prescriptions per 1,000 pregnant women) with the FDA warning in place, minus the estimated prescribing rate without the FDA warning, in October 2004. The estimated prescription rate without the FDA warning was based on extrapolating pre-warning trends in antidepressant prescribing.
Represents the linear rate of change (slope) in antidepressant prescribing during the post-warning period. The negative values (with 95% confidence intervals [CIs]) are rates of decrease, expressed as number of prescriptions per 1,000 pregnant women per month.
We estimated the immediate early effect of the FDA pregnancy warning on antidepressant prescribing by estimating the difference in prescribing rates with or without the FDA warning in October 2004, the first post-warning month, assuming that the FDA warning was the only factor influencing the change in prescribing pattern at that time point. There was a significant immediate effect of the FDA pregnancy warning on both overall antidepressant and SSRI prescribing (Table 3), suggesting that the pregnancy warning was associated with reduced antidepressant prescribing in general, and SSRI prescribing in particular, early in the post-warning period.
Sensitivity Analyses
Figure 2B shows results of the interrupted time-series analysis that estimated the combined effect of U.S. and Canadian pregnancy and suicide warnings using an expanded transition period (January 1, 2004 to October 31, 2004). Patterns of pre-warning increases and post-warning declines were nearly identical to those observed in the primary analysis for overall antidepressant, SSRI and non-SSRI prescribing (Table 4). Post-warning declines were also similar in magnitude to those observed in the main analysis (Table 4).
Table 4.
Relationship between pregnancy and suicide warnings and rates and trends of antidepressant prescribing (per 1,000 persons) during pregnancy, 2002–2005
| Antidepressant prescribing at the start of pre- warning period, N prescriptions per 1,000 womena | Rate of change in antidepressant use (pre-warning period), N prescriptions per 1,000 women/month (95% CI)b | Rate of change in antidepressant use (post-warning period), N prescriptions per 1,000 women/month (95% CI)c | |
|---|---|---|---|
| Any antidepressant | 34.17 | 0.51 (0.38, 0.64) | −1.00 (−1.58, −0.43) |
| SSRI | 25.40 | 0.26 (0.15, 0.36) | −0.66 (−1.14, −0.19) |
| Non-SSRI | 8.77 | 0.25 (0.20, 0.30) | −0.34 (−0.55, −0.12) |
Represents the estimated number of antidepressant prescriptions per 1,000 pregnant women in January 2004.
Represents the rate of change (slope) in antidepressant prescribing during the pre-warning period (January 1, 2002 – December 31, 2003). The positive values (with 95% confidence intervals [CIs]) are rates of increase, expressed as number of prescriptions per 1,000 pregnant women per month.
Represents the rate of change (slope) in antidepressant prescribing during the post-warning period (November 1, 2004 – July 31, 2005). The negative values (with 95% confidence intervals [CIs]) are rates of decrease, expressed as number of prescriptions per 1,000 pregnant women per month.
Age-stratified analyses also showed similar pre-warning increases and post-warning declines in overall antidepressant, SSRI, and non-SSRI prescribing for cohort members aged > 24 years and those between the ages of 18 and 24 years (Supplemental Table 2). Antidepressant prescribing rates were very low for pediatric cohort members (<18 years old) (Supplemental Table 2). In this subgroup, there were only minor increases in antidepressant prescribing during both the pre- and post-warning periods, and monthly changes in antidepressant prescribing rates were not statistically significant (Supplemental Table 2).
Discussion
In this large cohort of pregnant women, antidepressant prescribing increased between 1995 and early 2004, followed by sharp declines after the release of U.S. and Canadian public health advisory warnings about antidepressant-associated risk of perinatal complications. Expanding the transition period to include suicide risk warnings that appeared in close proximity with the pregnancy warnings did not change the pre- or post-warning antidepressant prescribing trends. Antidepressant prescribing trends within age strata not directly addressed by the suicide risk warnings were also consistent with those observed in the main analysis. Taken together, our results suggest that the 2004 release of public health advisory warnings about the risk of perinatal complications with antidepressants impacted antidepressant prescribing to pregnant women in Tennessee Medicaid.
Investigations of the effect of regulatory actions on antidepressant prescribing to pregnant women are important given how commonly antidepressants are used. Additionally, there exists natural tension between the increased recognition of the risks to mother and infant imposed by untreated gestational depression (including pre-eclampsia, preterm delivery, low birth weight, sudden infant death, developmental delay in offspring, post-partum depression, and maternal suicide) (Alder et al. 2007; Davalos et al. 2012; Lindahl et al. 2005; Milgrom et al. 2008), the primary indication for which antidepressant treatment would be considered during pregnancy, and lingering uncertainty regarding the balance of risks versus benefits of pre-partum antidepressant use (Payne and Meltzer-Brody 2009), making it difficult to evaluate the appropriateness of antidepressants during pregnancy (Kuehn 2009). Under these circumstances, patients and prescribers may place greater focus on the potential for adverse outcomes related to pharmacotherapy (Wisner et al. 2009b), and may be more sensitive to regulatory actions that warn of potential risks to the neonate.
Antidepressants are established treatment options for mood and anxiety disorders, and there is evidence that antidepressant use in pregnancy can reduce the risk of antenatal depressive relapses and post-partum depression (Cohen et al. 2006; Wisner et al. 2004), although not all studies are in agreement (Yonkers et al. 2011). It remains that case that the effectiveness of antidepressant treatment in the setting of pregnancy has received only limited investigation (Yonkers et al. 2009), and reports of adverse neonatal effects associated with fetal antidepressant exposure (Udechuku et al. 2010), including potential risk of cardiovascular malformations (Wurst et al. 2010) and persistent pulmonary hypertension (‘t Jong et al. 2012), have raised increasing concerns about the reproductive safety of SSRIs and other antidepressants. While the absolute risk of cardiac malformations or other birth defects associated with fetal SSRI exposure is thought to be very small (Alwan et al. 2007; Louik et al. 2007; ‘t Jong et al. 2012), uncertainty and dread regarding such outcomes may strongly influence the perception of risk among prescribers, patients, and their caregivers (Lyerly et al. 2007).
In recognition of these difficulties, the 2004 FDA advisory acknowledged potential risks associated with discontinuing antidepressants during pregnancy, and did not recommend avoiding antidepressants in this population. This raised the possibility that the advisory warnings could have little impact on antidepressant prescribing rates during pregnancy. Wichman and colleagues (2008) documented increased SSRI prescribing in pregnancy from 1993 to 2004, consistent with our findings and other studies also documenting increases in antidepressant use in pregnant women in the last decade (Andrade et al. 2008; Bakker et al. 2008; Cooper et al. 2007). In contrast with our results, there was no subsequent decline in antidepressant prescribing after 2004 (Wichman et al. 2008), suggesting that the regulatory warnings had modest impact on antidepressant use in the study population. These results could be considered broadly consistent with prior research showing that, in some cases, regulatory actions addressing potentially serious medication safety concerns had little impact on drug prescribing (Karpel et al. 2009; Ricci et al. 2009; Sclar et al. 2012). However, the Wichman et al. (2008) study only included women enrolled at a single medical center. In a very large retrospective cohort study of pregnant women in the U.S. who were Medicaid enrollees in 47 states (2000–2007), 8.1% were dispensed an antidepressant during pregnancy (Huybrechts et al., 2013). No significant change in the rate of antidepressant prescribing was associated with regulatory warnings concerning first trimester risk of congenital malformations with paroxetine (December 2005) or SSRI exposure and the risk of persistent pulmonary hypertension of the newborn (July 2006). However, issuance of the first FDA advisory warning about paroxetine and the risk of suicidality in children and adolescents (June 2003) was associated with a significant decrease in SSRI use during pregnancy. The relationship between the June 2004 release of FDA public health advisory warnings about the risk of perinatal complications with SSRIs and other antidepressants and antidepressant prescribing during pregnancy was not a specific focus of this report.
Alternatively, the unclear balance between risks and benefits for antidepressants in pregnancy and the potentially severe and permanent nature of many adverse neonatal outcomes linked with in utero antidepressant exposure could all contribute to heightened anxiety about antenatal antidepressant treatment. Under this circumstance, regulatory warnings targeting antidepressants in pregnancy could be plausibly associated with rapid declines in antidepressant prescribing, even if they do not recommend specifically against their use. Accordingly, we observed declines in antidepressant prescribing during the early post-warning period that persisted until the end of the study period.
To our knowledge, this was one of the largest studies examining the effect of regulatory warnings about antidepressant safety during pregnancy on longitudinal antidepressant prescribing trends in pregnant women. Large, automated medical encounter databases such as the one used in this study are valuable data sources for retrospective studies of programs or policies that may affect medication use (Ray 1997), particularly for relatively under-studied patient subgroups such as pregnant women. The large cohort size allowed precise estimation of antidepressant prescribing in the non-stratified analyses. Database prescription records provided objective, detailed, and low-cost measures of drug exposure that are not subject to recall bias (Ray and Griffin 1989) and correspond well with patient self-report of medication use (Johnson and Vollmer 1991; Landry et al. 1988; West et al. 1995). The interrupted time series design is considered the standard for evaluating policy changes that are unfeasible to investigate using randomized trials (Wagner et al. 2002).
There are also limitations to consider. First, our cohort, although large, consisted of Tennessee Medicaid beneficiaries, which may limit the generalizability of our results. Second, we could not verify that the prescribed antidepressants were actually taken, which is less of a concern for this study given our focus on antidepressant prescribing, rather than medication use. Third, regulatory warnings about antidepressant-associated suicidal behavior could have influenced the estimated effect of the pregnancy warnings on antidepressant prescribing. We believe that such an effect would be small based on results of the sensitivity analyses, the relatively targeted effects of the FDA suicide warnings on children and adolescents (Olfson et al. 2008), and the fact that the suicide warnings did not involve and concerns related specifically to pregnancy or neonatal outcomes. Nevertheless, effects of the pregnancy and suicide warnings could not be evaluated separately. Fourth, we used a single-arm time-series design that used the level and trend of the pre-warning segment as a non-concurrent control for the post-warning segment. Although single-arm interrupted time-series are considered methodologically acceptable for investigating the effects of regulatory actions (Wagner et al. 2002), we cannot be certain that extrapolation of pre-intervention trend accurately represents the counterfactual rate of antidepressant prescribing had the regulatory warnings never occurred. Finally, we did not quantify antidepressant prescribing in specific patient subgroups (e.g., new antidepressant users), or by therapeutic indication, prescriber specialty, or disease severity. We did not restrict our cohort to women with diagnosed depression based on antidepressant prescriptions and ICD-9 diagnosis codes because antidepressants are often used for indications other than unipolar depressive disorders, and ICD-9 codes indicating a clinical diagnosis of depressive disorders were not validated against a diagnostic gold standard.
Conclusions
In a large cohort of pregnant women, antidepressant prescribing increased steadily between 1995 and early 2004. The late 2004 release of public health advisories about antidepressant-associated risk of perinatal complications in the U.S. and Canada was associated with steady decreases in antidepressant prescribing that persisted until the end of the study period. These results suggest that the advisory warnings were impactful on prescribing of antidepressants to pregnant women in our cohort.
Supplementary Material
Antidepressant prescribing trends during pregnancy (1995–2007), showing U.S. and Canadian pregnancy and suicide warning issue dates.
Footnotes
Financial support/disclosures: This work was supported by grants from the National Institutes of Health K24 AI77930U01, HL 072471, RC4 MH092755, and R03 MH088902. R.C.S. received research funding from Bristol-Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Forest Pharmaceuticals, Janssen Pharmaceutica, Novartis Pharmaceuticals, Otsuka Pharmaceuticals, Pamlab, Pfizer, Repligen, and St. Jude Medical. R.C.S. has consulted for Eli Lilly and Company, Cyberonics, Evotec AG, Forest Pharmaceuticals, Gideon Richter PLC, Janssen Pharmaceutica, Medronic, Otsuka Pharmaceuticals, Pamlab, Inc, Pfizer, Repligen, and Sierra Neuropharmaceuticals. The remaining authors report no conflict of interest. We are indebted to the Tennessee Bureau of TennCare of the Department of Finance and Administration, and the Tennessee Department of Health, Office of Policy, Planning & Assessment, for providing the data on the TennCare cohort.
References
- Alder J, Fink N, Bitzer J, Hosli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. Journal of Maternal-Fetal and Neonatal Medicine. 2007;20:189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2008;356:2684–2692. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Willy ME, Staffa JA, Platt R. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198:194–195. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Bakker MK, Kolling P, van den Berg PB, de Walle HE, de Jong van den Berg LT. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol. 2008;65:600–606. doi: 10.1111/j.1365-2125.2007.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Whybrow PC, Angst J, Versiani M, Moller HJ. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Unipolar Depressive Disorders, Part 1: Acute and continuation treatment of major depressive disorder. World J Biol Psychiatry. 2002;3:5–43. doi: 10.3109/15622970209150599. [DOI] [PubMed] [Google Scholar]
- Becker RA, Chambers JM, Wilks AR. The New S Language. Wadsworth & Brooks/Cole; 1988. [Google Scholar]
- Bilszta JL, Tsuchiya S, Han K, Buist AE, Einarson A. Primary care physician’s attitudes and practices regarding antidepressant use during pregnancy: a survey of two countries. Arch Womens Ment Health. 2011;14:71–75. doi: 10.1007/s00737-010-0197-8. [DOI] [PubMed] [Google Scholar]
- Bonari L, Pinto N, Ahn E, Einarson A, Steiner M, Koren G. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49:726–35. doi: 10.1177/070674370404901103. [DOI] [PubMed] [Google Scholar]
- Canadian Psychiatric Association. Clinical practice guidelines. Management of anxiety disorders. Can J Psychiatry. 2006;51:9S–91S. [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- Cleveland WS. LOWESS: a program for smoothing scatterplots by robust locally weighted regression. The American Statistician. 1981;35:54. [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- Committee on Safety of Medicines’ Expert Working Group. Report of the CSM Expert Working Group on the safety of selective serotonin reuptake inhibitor antidepressants. 2003 http://www.mhra.gov.uk/home/groups/pl-p/documents/drugsafetymessage/con019472.pdf.
- Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196:544–45. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Womens Ment Health. 2012;15:1–14. doi: 10.1007/s00737-011-0251-1. [DOI] [PubMed] [Google Scholar]
- Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry. 2010;71(Suppl E1):e04. doi: 10.4088/JCP.9058se1c.04gry. [DOI] [PubMed] [Google Scholar]
- Einarson TR, Einarson A. Newer antidepressants in pregnancy and rates of major malformations: a meta-analysis of prospective comparative studies. Pharmacoepidemiol Drug Saf. 2005;14:823–27. doi: 10.1002/pds.1084. [DOI] [PubMed] [Google Scholar]
- Einarson A, Schachtschneider AK, Halil R, Bollano E, Koren G. SSRI’S and other antidepressant use during pregnancy and potential neonatal adverse effects: impact of a public health advisory and subsequent reports in the news media. BMC Pregnancy Childbirth. 2005;5:11. doi: 10.1186/1471-2393-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. FDA Statement on Recommendations of the Psychopharmacologic Drugs and Pediatric Advisory Committees. 2004 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108532.htm.
- Hayes RM, Wu P, Shelton RC, Cooper WO, Dupont WD, Mitchel E, Hartert TV. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am J Obstet Gynecol. 2012;207:49.e1–9. doi: 10.1016/j.ajog.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. Health Canada advises of potential serious adverse effects of SSRIs and other antidepressants on newborns [advisory] Ottawa: Health Canada; 2004. [Google Scholar]
- Hemels ME, Einarson A, Koren G, Lanctot KL, Einarson TR. Antidepressant use during pregnancy and the rates of spontaneous abortions: a meta-analysis. Ann Pharmacother. 2005;39:803–09. doi: 10.1345/aph.1E547. [DOI] [PubMed] [Google Scholar]
- Huybrechts KF, Palmsten K, Mogun H, Kowal M, Avorn J, Steoguchi-Iwata S, Hernandez-Diaz S. National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry. 35:265–71. doi: 10.1016/j.genhosppsych.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD) Int J Neuropsychopharmacol. 2012;15:825–40. doi: 10.1017/S1461145711001209. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Vollmer WM. Comparing sources of drug data about the elderly. J Am Geriatr Soc. 1991;39:1079–84. doi: 10.1111/j.1532-5415.1991.tb02872.x. [DOI] [PubMed] [Google Scholar]
- Karpel JP, Peters JI, Szema AM, Smith B, Anderson PJ. Differences in physicians’ self-reported knowledge of, attitudes toward, and responses to the black box warning on long-acting beta-agonists. Ann Allergy Asthma Immunol. 2009;103:304–10. doi: 10.1016/S1081-1206(10)60529-7. [DOI] [PubMed] [Google Scholar]
- Koren G, Nordeng H. Antidepressant use during pregnancy: the benefit-risk ratio. Am J Obstet Gynecol. 2012;207:157–63. doi: 10.1016/j.ajog.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31:206–19. doi: 10.1016/j.genhosppsych.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. No easy answers for physicians caring for pregnant women with depression. JAMA. 2009;302:2413–4. 2420. doi: 10.1001/jama.2009.1762. [DOI] [PubMed] [Google Scholar]
- Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, Parikh SV, Patten SB, Ravindran AV Canadian Network for Mood and Anxiety Treatments (CANMAT) Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord. 2009;117(Suppl 1):S26–S43. doi: 10.1016/j.jad.2009.06.041. [DOI] [PubMed] [Google Scholar]
- Landry JA, Smyer MA, Tubman JG, Lago DJ, Roberts J, Simonson W. Validation of two methods of data collection of self-reported medicine use among the elderly. Gerontologist. 1988;28:672–76. doi: 10.1093/geront/28.5.672. [DOI] [PubMed] [Google Scholar]
- Lanni C, Gonovi S, Lucchelli A, Boselli C. Depression and antidepressants: molecular and cellular aspects. Cell Mol Life Sci. 2009;66:2985–3008. doi: 10.1007/s00018-009-0055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8:77–87. doi: 10.1007/s00737-005-0080-1. [DOI] [PubMed] [Google Scholar]
- Louik C, Lin AE, Werler MM, Hernandez-Diaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356:2675–83. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- Lyerly AD, Mitchell LM, Armstrong EM, Harris LH, Kukla R, Kuppermann M, Little MO. Risks, values, and decision making surrounding pregnancy. Obstet Gynecol. 2007;109:979–84. doi: 10.1097/01.AOG.0000258285.43499.4b. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, Ericksen J, Ellwood D, Buist A. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108:147–57. doi: 10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Olfson MM, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66:848–56. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Druss BG. Effects of Food and Drug Administration warnings on antidepressant use in a national sample. Arch Gen Psychiatry. 2008;65:94–101. doi: 10.1001/archgenpsychiatry.2007.5. [DOI] [PubMed] [Google Scholar]
- Payne JL, Meltzer-Brody S. Antidepressant use during pregnancy: current controversies and treatment strategies. Clin Obstet Gynecol. 2009;52:469–82. doi: 10.1097/GRF.0b013e3181b52e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos E, Oraichi D, Rey E, Blais L, Berard A. Prevalence and predictors of antidepressant use in a cohort of pregnant women. BJOG. 2007;114:1055–64. doi: 10.1111/j.1471-0528.2007.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WA. Policy and program analysis using administrative databases. Ann Intern Med. 1997;127:712–18. doi: 10.7326/0003-4819-127-8_part_2-199710151-00055. [DOI] [PubMed] [Google Scholar]
- Ray WA, Griffin MR. Use of Medicaid data for pharmacoepidemiology. Am J Epidemiol. 1989;129:837–49. doi: 10.1093/oxfordjournals.aje.a115198. [DOI] [PubMed] [Google Scholar]
- Ricci JR, Coulen C, Berger JE, Moore MC, McQueen A, Jan SA. Prescriber compliance with black box warnings in older adult patients. Am J Manag Care. 2009;15:e103–e108. [PubMed] [Google Scholar]
- Sclar DA, Robison LM, Castillo LV, Schmidt JM, Bowen KA, Oganov AM, Skaer TL, Kogut SJ. Concomitant use of triptan, and SSRI or SNRI after the US Food and Drug Administration alert on serotonin syndrome. Headache. 2012;52:198–203. doi: 10.1111/j.1526-4610.2011.02067.x. [DOI] [PubMed] [Google Scholar]
- Shadish WR, Cook TD, Campbell T. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Boston: Houghton Mifflin; 2002. [Google Scholar]
- ‘t Jong GW, Einarson T, Koren G, Einarson A. Antidepressant use in pregnancy and persistent pulmonary hypertension of the newborn (PPHN): a systematic review. Reprod Toxicol. 2012;34:293–97. doi: 10.1016/j.reprotox.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Udechuku A, Nguyen T, Hill R, Szego K. Antidepressants in pregnancy: a systematic review. Aust N Z J Psychiatry. 2010;44:978–96. doi: 10.3109/00048674.2010.507543. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Authority. FDA Medwatch drug alert on Effexor and SSRIs. Jun 3, 2004. [Google Scholar]
- Ververs T, Kaasenbrood H, Visser G, Schobben F, de Jong-van den Berg, Egberts T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. 2006;62:863–70. doi: 10.1007/s00228-006-0177-0. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142:1103–12. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- Wichman CL, Fothergill A, Moore KM, Lang TR, Heise RH, Jr, Watson WJ. Recent trends in selective serotonin reuptake inhibitor use in pregnancy. J Clin Psychopharmacol. 2008;28:714–16. doi: 10.1097/JCP.0b013e31818b53fd. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, Perel JM, Jones-Ivy S, Bodnar LM, Singer LT. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009a;166:557–66. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Appelbaum PS, Uhl K, Goldkind SF. Pharmacotherapy for depressed pregnant women: overcoming obstacles to gathering essential data. Clin Pharmacol Ther. 2009b;86:362–65. doi: 10.1038/clpt.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RL. Prevention of postpartum depression: a pilot randomized clinical trial. Am J Psychiatry. 2004;161:1290–92. doi: 10.1176/appi.ajp.161.7.1290. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The health of youth. Geneva: World Health Organization; 1989. Report No.: Document 399 A42/Technical Discussions/2. [Google Scholar]
- Wurst KE, Poole C, Ephross SA, Olshan AF. First trimester paroxetine use and the prevalence of congenital, specifically cardiac, defects: a meta-analysis of epidemiological studies. Birth Defects Res A Clin Mol Teratol. 2010;88:159–70. doi: 10.1002/bdra.20627. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Gotman N, Smith MV, Forray A, Belanger K, Brunetto WL, Lin H, Burkman RT, Zolop CM, Lockwood CJ. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology. 2011;22:848–54. doi: 10.1097/EDE.0b013e3182306847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114:703–13. doi: 10.1097/AOG.0b013e3181ba0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antidepressant prescribing trends during pregnancy (1995–2007), showing U.S. and Canadian pregnancy and suicide warning issue dates.