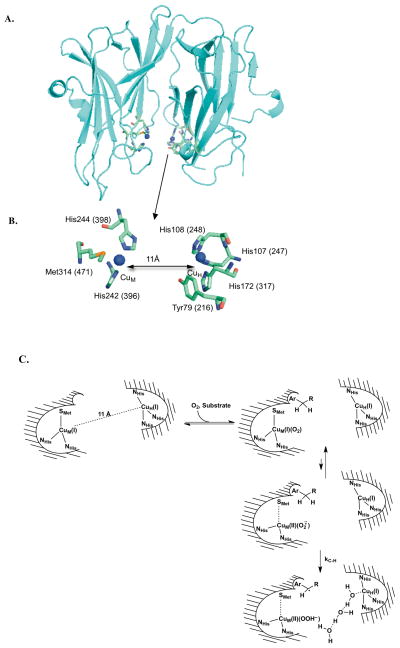
Figure 1.
A. X-ray crystal structure of the catalytic core of peptidylglycine α-hydroxylating monooxygenase (PDB: 1PHM) (5). B. The active site is enlarged to show the coordinating ligands to CuM (H242, H244, and M314) and CuH (H107, H108, and H172) and the conserved tyrosine (Y79 in PHM and Y216 in TβM) that is the focus of this study. Residue numbers are shown for PHM (without parentheses) and TβM (with parentheses). C. Consensus mechanism through the first irreversible step (22, 24). Waters are visualized in the last frame only, to illustrate a possible conduit for proton-coupled electron transfer from CuH to CuM.