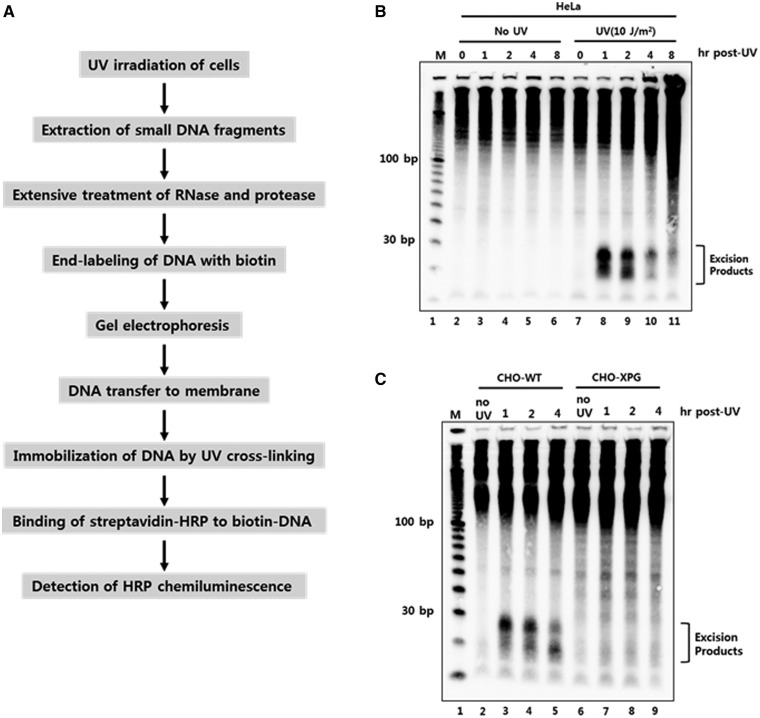
Figure 1.
Development of a novel non-radioisotopic method for the detection of excised oligonucleotide products of nucleotide excision repair in vivo. (A) Schematic representation for the detection of excised oligonucleotides containing UV damage in human cells. UV-irradiated cells are harvested and subjected to DNA extraction to isolate low molecular weight DNAs. After extensive treatment with RNase and protease, the short DNA molecules are labeled with biotin at the 3′-end using terminal deoxynucleotidyl transferase (TdT). The biotin-labeled oligonucleotides are separated by gel electrophoresis and then transferred to a nylon membrane. The transferred biotin-DNA molecules are immobilized by UV cross-linking, incubated with HRP-conjugated streptavidin, and detected with a chemiluminescence reagent. (B) HeLa cells were exposed to 10 J/m2 of UV-C and then harvested at different time points. The cells were subjected to the modified Hirt lysis and labeling procedures as in A. The DNA marker (M) is a 10 bp DNA ladder that was labeled with biotin and loaded onto the gel. (C) CHO wild-type (AA8) and XPG-deficient (UV135) cells were harvested at different times after UV exposure to 10 J/m2 of UV-C. Excised oligonucleotides containing UV damage were then extracted and processed as described in A.