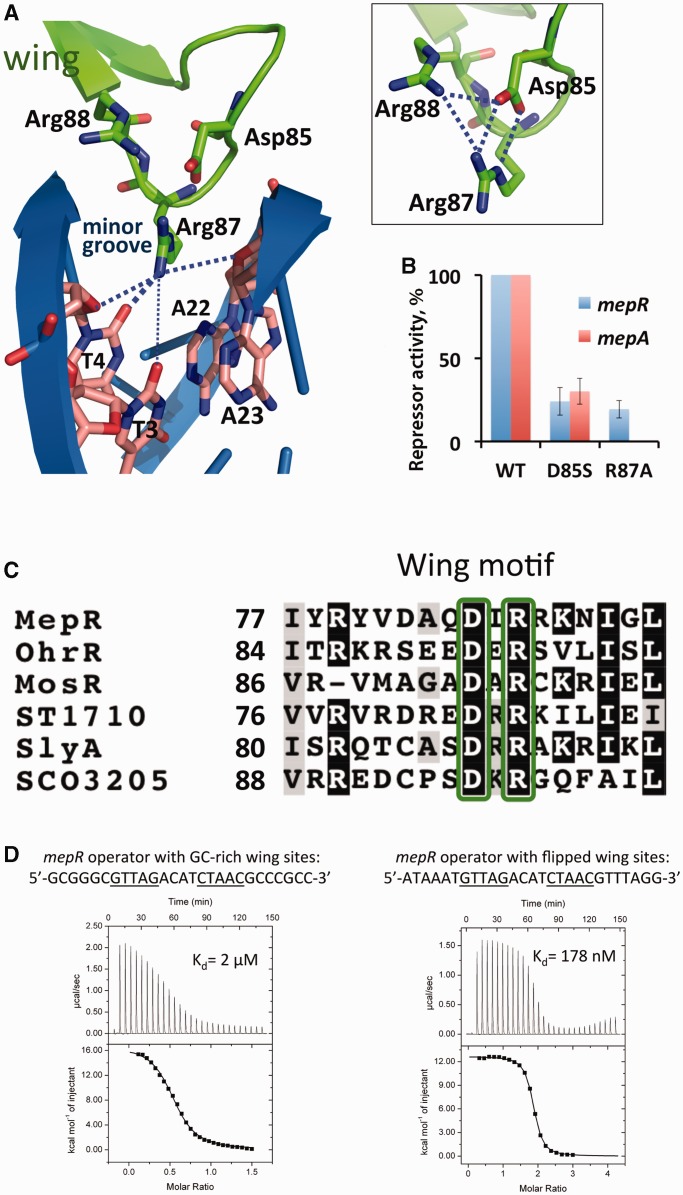
Figure 4.
Interaction of the wing motif with the minor groove. (A) Residue Arg87 interaction with the O2 atoms of T4 and T3, and the ribosyl oxygens of T4 and A23. The boxed inset shows the electrostatic network between residues Arg87, Asp85 and Arg88. (B) Repression of the chromosomal mepR or mepA operators by MepR mutants D85S and R87A as determined by the ability of mutant MepR to affect the expression of the mepR::lacZ and mepA::lacZ transcriptional fusions. Data are presented as the mean repressor activity ± the standard deviation. The data were normalized to WT MepR expression and are provided in Supplementary Table S2. (C) Sequence alignment of the wing motif region of MarR proteins the structures of which have been solved in complex with cognate DNA. The conserved residues Asp85 and Arg87 are shown in green boxes. The alignment was created using ClustalW (45) and shaded using BoxShade 3.2. (D) MepR binding to the mepR operator with its wing-binding nucleotides modified. The sequences of oligodeoxynucleotides are indicated above the thermograms. In the left panel, DNA (400 μM) was injected into protein (55 μM); a stoichiometry of 0.5 corresponds to a single dsDNA molecule binding to a dimer of MepR. In the right panel, protein (400 μM) was injected into DNA (20 μM); a stoichiometry of 2 corresponds to 1 dimer of MepR binding to one dsDNA. The fitted thermodynamic parameters are provided in Supplementary Table S1.