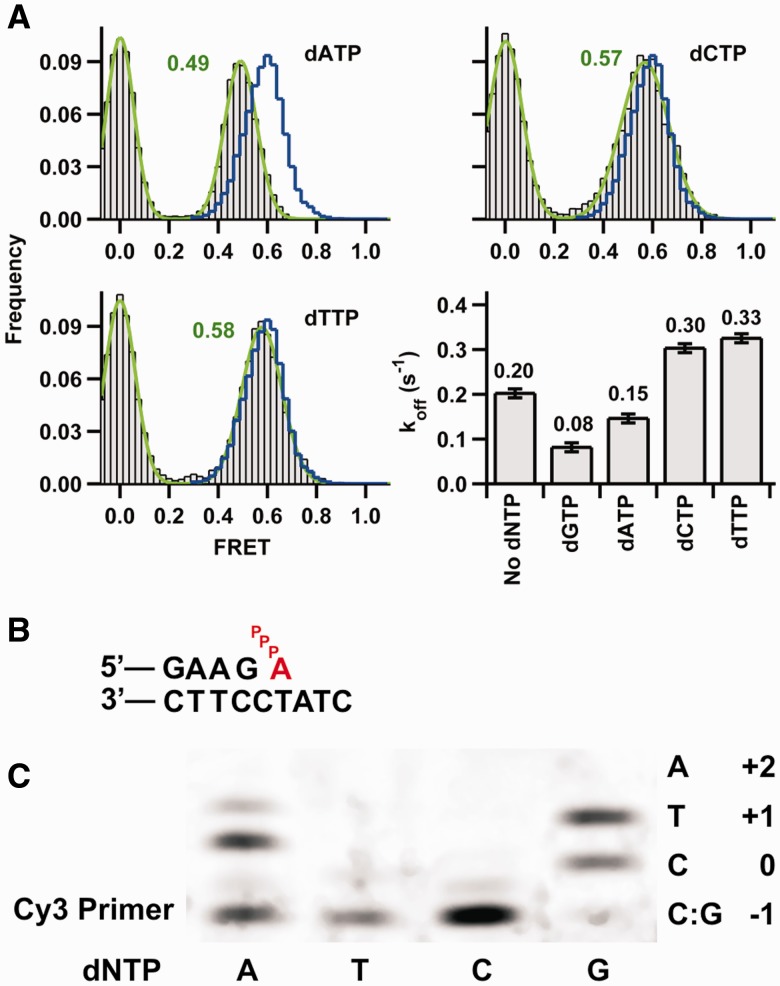
Figure 5.
Conformational changes associated with incorrect nucleotide binding. (A) SmFRET histograms for Dpo4 binding 8Cy3 duplex in the presence of each of the three incorrect nucleotides shown in gray. Ternary complex histograms with the correct nucleotide dGTP are shown as a blue line and are the same as in Figure 3. Dissociation constant koff (calculated as in Figure 1C) in the absence and presence of the indicated dNTP. The concentration of correct nucleotide dGTP was 200 μM, and concentration of the incorrect dNTPs was 1 mM. The reported errors were determined from the mono-exponential fits to the dwell-time distributions. (B) Proposed misaligned template arrangement for the ternary complex with an incoming dATP pairing with the +1 T in the template. (C) Single-nucleotide incorporations by Dpo4. Reactions were carried out at 50°C in buffer containing 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 50 mM NaCl, 0.025 mg/ml bovine serum albumin, 5 nM primer template (24mer/33mer) and 10 nM Dpo4. Reactions were initiated by the addition of dNTPs (final concentration 500 µM) and incubated for 10 min. The local template sequence is shown to the right of the gel.