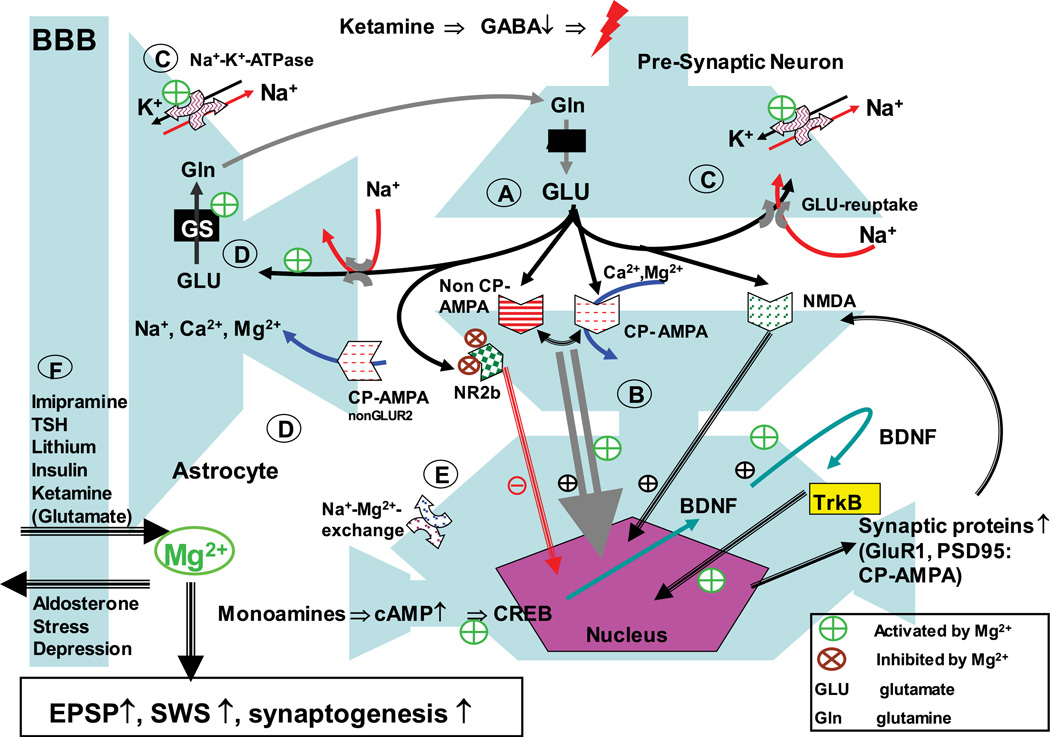
Figure 1.
Magnesium involvement in ketamine-induced pathways. Ketamine administration leads to a cascade of events finally resulting in modifications of glutamatergic receptor profile and synaptogenesis. Functional consequences are increased excitatory postsynaptic potentials (EPSP) and increased slow-wave sleep; both phenomena are also induced by Mg2+. In detail: (A) ketamine inhibits GABAergic interneurons and therefore activates the release of glutamate downstream in the context of partial NR2b receptor blockade. (B) AMPA mediates BDNF expression and release. BDNF activates TrkB receptor, which induces changes in gene expression. BNDF induces its own expression. NMDA receptor activation is facilitating this process.33 This could constitute a feedforward mechanism, explaining the long-term effect of ketamine administration. Further, the expression of synaptic proteins is induced, in particular GluR1 and PSD-95, which constitute the synaptic expression of Ca2+-permeable AMPA receptors (CP-AMPA).34 Importantly, CP-AMPA are permeable for Mg2+. (C) Glutamate is taken up quickly by neurons and astrocytes. This is of importance as high concentrations of glutamate can spill over to extrasynaptic NMDA receptors, which appear to be primarily from the NR2b type. Their activation can block synaptogenesis and lead to cell damage. Rapid reuptake of glutamate prevents this spillover. Glutamate reuptake is an energy-dependent process driven by the Na+-gradient over the membrane,35 which in itself is driven by the Na+–K+–ATPase. The Na+–K+–ATPase is dependent on Mg2+, therefore increased Mg2+ availability supports its activity and secondarily glutamate clearance. (D) In parallel glutamate receptors at astrocytes are activated. CP-AMPA receptor activation can lead to an increase in astrocytic Ca2+35 and Mg2+36 A potential consequence of increased Mg2+ in astrocytes is activation of glutamine synthetase (GS), which is Mg2+ dependent.37,38 (E) A neuronal Na+–Mg2+–exchange mechanism regulates intracellular Mg2+ concentration. Imipramine appears to block Na+–Mg2+ exchange, preventing the efflux of Mg2+ from the neuron. (F) Magnesium uptake into the brain has been described with compounds, which are known to be efficacious in treatment-refractory depression, i.e., ketamine (via its glutamate-releasing capability), TSH, lithium, imipramine, and potentially an insulin-related mechanism (metformin, glitazones). On the other hand, stress and refractory depression are linked to lower Mg2+ levels in the brain, which may in part be mediated via an aldosterone mediated mechanism. Adapted with permission from Ref. 11.