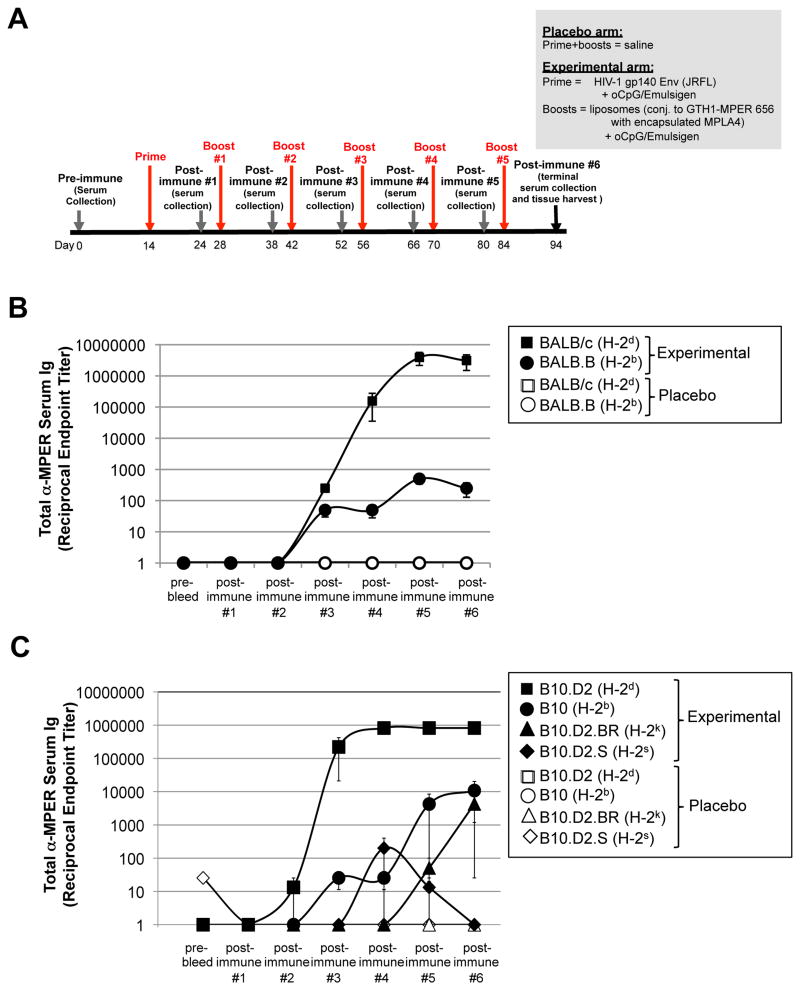
Figure 1. MHC-dependent MPER+ Ab responses are observed in two independent series of MHC haplotype congenic mouse strains.
A. Experimental design of immunization studies. Shown are the placebo (saline) and experimental (JRFL prime, [TLR4/9-MPER peptide-liposome] conjugate immunogen boost) arms and the study schedule, including timing of prime/boosts, serum collections, and harvests. Note that because the highest peak MPER+ Ab titers were observed in all strains using the experimental regimen shown (i.e. relative to other adjuvant/immunogen combinations tested; Table 1, 2), it is used in all other results in this study (i.e., unless otherwise noted). B. ELISA measurements of total MPER (2F5 epitope)-specific serum Ig (kappa) responses (mean±SEM) in BALB/c and BALB.B congenic mice, were measured against plate-bound 2F5 nominal epitope-containing peptide SP62, and calculated as reciprocal endpoint titers, as previously described (28) and in the Materials and Methods. Data are shown as mean±SEM reciprocal endpoint titers of individual mouse serum samples at various serum collection time points (using >3 individual mice/time point) during course of immunization protocol. C. ELISA measurements of MPER-specific serum Ig levels in a B10.D2 series of MHC haplotype congenic mice, measured and represented as in Fig. 1B.