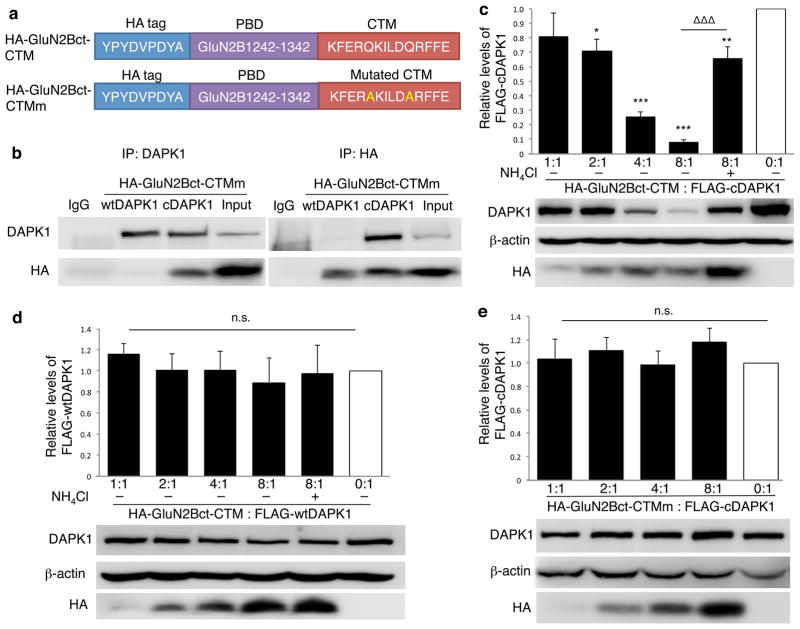
Figure 2. DAPK1 targeting peptide knocks down active DAPK1 in HEK cells.
(a) Linear representation of DAPK1-targeting peptide HA-GluN2Bct-CTM and its non-functional control peptide HA-GluN2Bct-CTMm. (b) Reciprocal co-immunoprecipitation followed by immunoblotting revealed that GluN2Bct specifically interacted with cDAPK1, but not wtDAPK1. Flag-tagged wild type (inactive) DAPK1 (wtDAPK1) or constitutively active mutant of DAPK1 (cDAPK1) was co-expressed with either HA-GluN2Bct-CTM or HA-GluN2Bct-CTMm at various ratios in HEK cells, and co-immunoprecipitation and/or immunoblotting was performed 24hrs thereafter. Anti-HA was used to detect HA-GluN2Bct-CTM and HA-GluN2Bct-CTMm, while anti-FLAG was used to detect wtDAPK and cDAPK. (c) HA-GluN2Bct-CTM specifically and dose-dependently decreased the level of cDAPK1 (n=4 independent experiments from 4 separate cell cultures and transfections; p<0.001; F(5,18)=18.27), but not wtDAPK1 (d; n=3 independent experiments from 3 separate cell cultures and transfections; p=0.933; F(5,12)=0.249). HA-GluN2Bct-CTM mediated cDAPK1 knockdown was significantly reduced by NH4Cl (c; 20mM; n=4;ΔΔΔ p<0.001, compared to HA-GluN2Bct-CTM:cDAPK1=8:1 group) and by mutational inactivation of CTM (e; HA-GluN2Bct-CTMm; p=0.785; F(4,35)=0.432, 8 independent experiments from 8 separate cell cultures and transfections). Levels of cDAPK1 or wtDAPK1 co-transfected with pcDNA3.0 vector (0:1, white bar) represent the control values arbitrarily set as 1. Membranes re-probed for β-actin were used as a loading control. One-way ANOVA was used with Fischer LSD. *p<0.05 ** p<0.01 ***p<0.001, compared with the control. Bars represent relative mean values±s.e.m. Full-length blots are presented in Supplementary Figure 9.