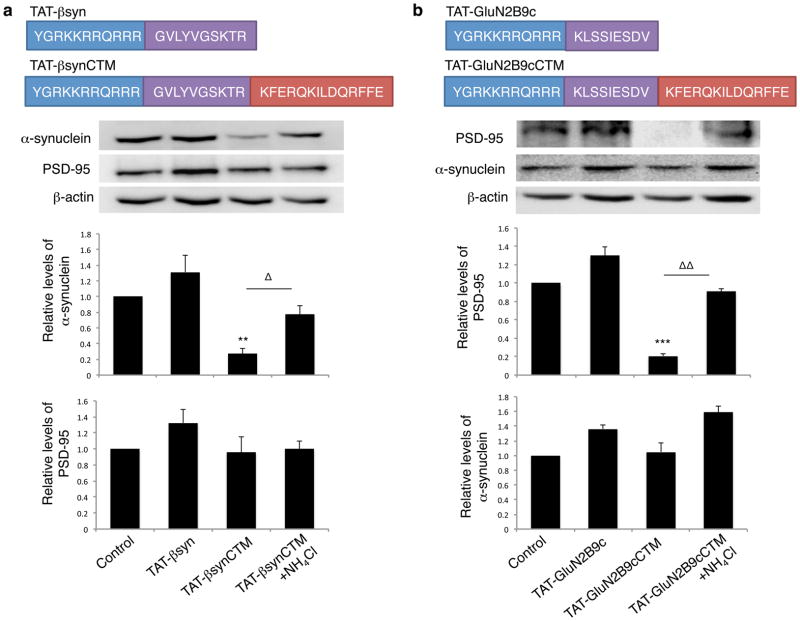
Figure 4. Target peptide-mediated respective degradation of α-synuclein and PSD-95 in cultured neurons.
(a) Top: Schematics of the synthetic cell-penetrating α-synuclein targeting peptide TAT-βsynCTM and its control TAT-βsyn. Middle: Immunoblots demonstrate that TAT-βsynCTM (25μM; n=5), but not the CTM-lacking control peptide TAT-βsyn (25μM; n=5), specifically decreased the targeted endogenous α-synuclein (One way-ANOVA Tukey post-hoc, p<0.001, F(3,16)=12.435), without affecting the level of unrelated control proteins PSD-95 at 4 hours (bottom), and this reduction was prevented in the presence of lysosomal inhibitor NH4Cl (20mM; n=5). Sample size represents individual experiments from at least 3 separate primary cultures. (b) Top: Schematics of PSD-95 targeting peptide TAT-GluN2B9cCTM and control TAT-GluN2B9c. Middle: TAT-GluN2B9cCTM (25μM; n=4), but not Tat-GluN2B9c (25μM; n=4), effectively degraded endogenous PSD-95 (One-way ANOVA Tukey post-hoc, p<0.001, F(3,12)=18.154) without perturbing untargeted protein α-synuclein (Bottom). NH4Cl rescued PSD-95 degradation. Sample size represents individual experiments from at least 2 separate primary cultures. Membrane re-probing for β-actin was used as additional specificity and loading controls. * p<0.05, **,ΔΔ p<0.01 and ***p<0.001; bars represent relative mean values±s.e.m. normalized to the naïve non-treated control (arbitrarily set as 1). Full length blots were presented in Supplementary Figure 9.