Figure 1.
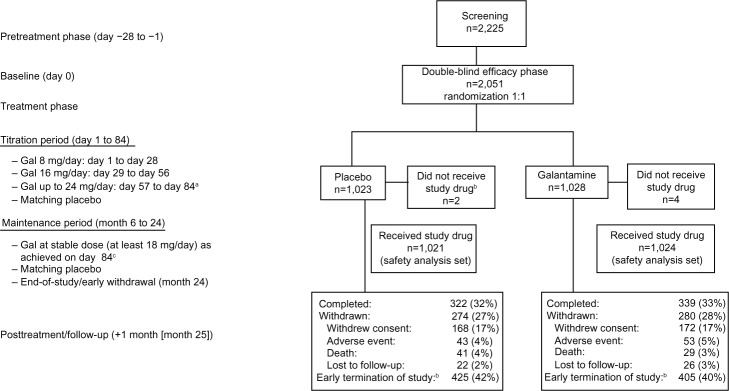
Patient disposition.
Notes: aUptitration (from 16 mg/day to 24 mg/day) or downtitration (from 24 mg/day to 16 mg/day) of dose was allowed, based on tolerability and the investigator’s judgment. Patients unable to tolerate a minimum of 16 mg/day dose were to discontinue treatment and were followed until the end of the maintenance and posttreatment period. The total number of patients included in the safety analysis set was n=2,045; bearly study termination, per Data Safety Monitoring Board recommendation, when the prespecified number of deaths was ascertained and a significant imbalance favoring galantamine was observed; ca one-time dose titration to 16 or 24 mg/day was allowed, based on the investigator’s judgment and patient tolerability.
Abbreviation: Gal, galantamine.
