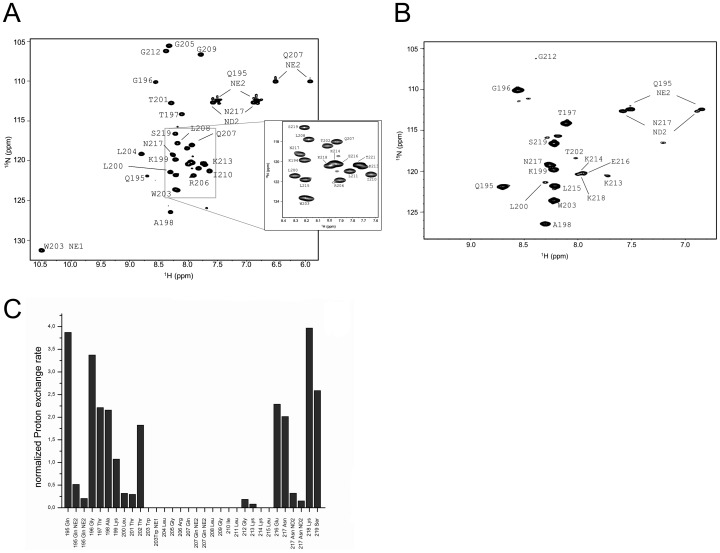
Figure 5. Water accessibility measurements of ErnsΔNΔC.
(A) 15N-HSQC, and (B) CLEANEX spectra of ErnsΔNΔC (Arg194 – Thr221), a 6 amino acid C-terminal truncated version of ErnsΔN, recorded in a bicelle system composed of DHPC/DMPC (4∶1) and a protein/lipid ratio of 1∶222. The 15N chemical shift is displayed on the y-axis and the 1H shift is shown on the x-axis. Identified NH groups are marked with the single letter code of the corresponding amino acids and numbered according to the full-length Erns protein. The central spectrum area in (A) is shown as a blow up in the box on the right for better clarity. The side chain NH groups of Trp203 and 222 are marked as NE1. The Gln207 side chain, together with the side chains of Gln195 and Asn217 could not be further assigned and are therefore marked as NE2 or ND2. (C) Calculated normalized proton exchange rates of all identified NH groups of the spectra. Lys220 and Thr221 could not be assigned unambiguously and therefore are not presented in the figure.