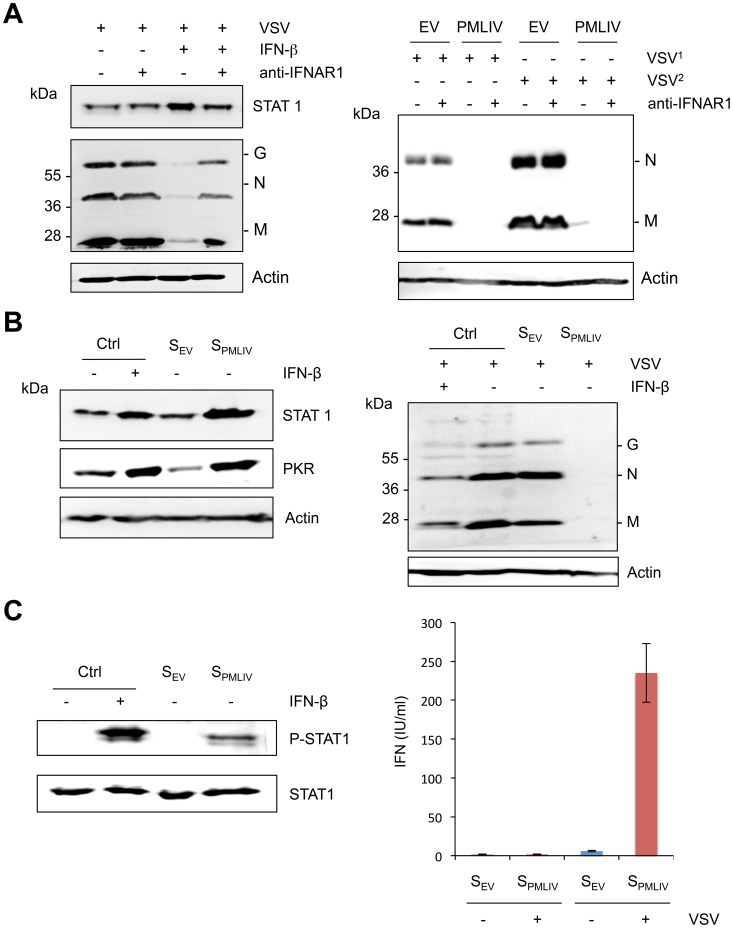
Figure 7. Intrinsic and innate immune properties of PMLIV.
(A) U373MG cells were left untreated or treated for 24 h with 100 units/ml of IFN-β in the absence or the presence of 20 µg/ml of purified anti-IFNAR1 mAb before infection with VSV at an MOI of 1 for 8 h (left panel). U373MG and U373MG-PMLIV cells were infected with VSV at an MOI of 0.2 for 8 h (VSV1) or 12 h (VSV2) in the absence or the presence of 20 µg/ml of anti-IFNAR1 mAb (right panel). Cell extracts were analyzed by Western blotting and revealed by antibodies directed against VSV, STAT1 or Actin. (B) Activity of secreted IFN in infected U373MG-PMLIV cells. Culture supernatants (from U373MG-EV cells [SEV] and U373MG-PMLIV cells [SPMLIV]) were treated with acid buffer to inactivate virus, and the pH was then neutralized. One series of HeLa cells were left untreated as a control (Ctrl) or treated for 24 h with 100 units/ml of IFN-β, SEV or SPMLIV, then their extracts were analyzed by Western blot for PKR and STAT1 (left panel). The second series of HeLa cells were infected with VSV at an MOI of 0.2 for 8 h and the cell extracts were analyzed by Western blotting using anti-VSV and anti-Actin antibodies (right panel). (C) Secreted IFN activates STAT1 phosphorylation (left panel). HeLa cells were left untreated (Ctrl) or treated for 30 min with 1000 units/ml of IFN-β, SEV or SPMLIV, then their extracts were analyzed by Western blot for P-STAT1 and STAT1. Quantification of produced type I IFN upon VSV infection (right panel). IFN was quantified using the reporter cell line HL116 that carries the luciferase gene under the control of the IFN-inducible 6–16 promoter [55]. HL116 cells were incubated for 8 h with a standard containing titrated human IFN-β, the supernatants from U373MG-EV [SEV] and U373MG-PMLIV [SPMLIV] cells infected with VSV at an MOI of 1 for 20 h or the supernatants from uninfected U373MG-EV and U373MG-PMLIV. Cells were then lysed and luciferase activity measured. IFN levels were expressed as equivalent of IFN-β concentration, in IU/ml. Means and standard deviations of two independent experiments are shown.