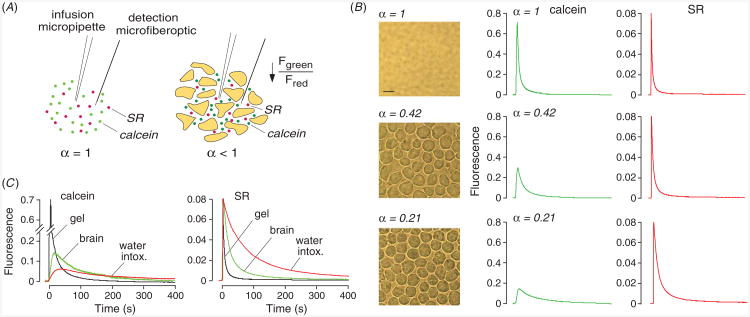
Figure 2.
Extracellular space volume determination by ‘pulsed-infusion microfiberoptic photodetection’ (PIMP). (A) Method principle. Two dyes—calcein at self-quenching concentration and sulforhodamine 101 (SR), a dye that does not undergo self-quenching—are deposited by a brief (1 s) iontophoresis pulse. Reduced extracellular space volume fraction (α) results in a reduced aqueous volume for dye deposition, and consequent reduced calcein fluorescence. SR fluorescence provides an α-independent ‘reference’. (B) Measurements in gels embedded with live SP2/0 cells in 0.3% agarose, giving α of 1 (gel not containing cells), 0.42 and 0.21. Left: brightfield micrographs of the cell-embedded gels. Right: time course of calcein (center panels) and SR (right panels) fluorescence following pulsed iontophoretic dye delivery. (C) PIMP measurement made at a depth of 400 μm in mouse brain cortex before and after inducing brain swelling by intraperitoneal water administration. For comparison data shown for (cell-free) agarose cel. Adapted from [18].