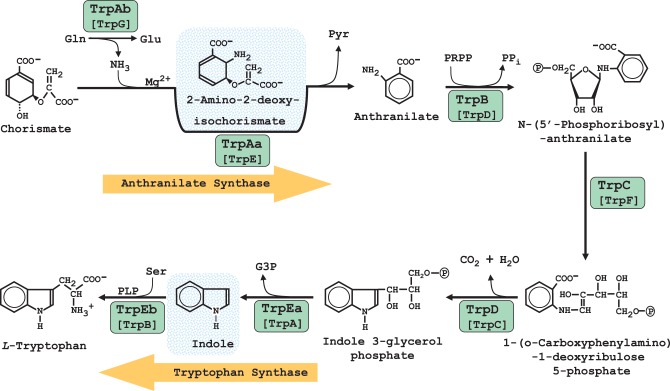
Figure 3.
Biochemical pathway of Trp biosynthesis. The green ovals encircle the logical acronym designations which are based upon the order of enzyme reactions (Xie et al., 2003). Below the latter are shown the classic acronym designations. Enzyme-bound intermediates for anthranilate synthase and tryptophan synthase are shown with blue shading. The initial pathway reaction is catalyzed by anthranilate synthase. The TrpAa subunit can catalyze the reaction as an aminase utilizing ammonia as a nitrogen donor, but the physiological reaction is carried out as an amidotransferase via the contribution of the TrpAb subunit which utilizes glutamine as the source of the ammonia reactant. Although tryptophan synthase is a two-subunit complex in which indole is an enzyme-bound intermediate, isolated TrpEb is capable of utilizing free indole.