FIGURE 1.
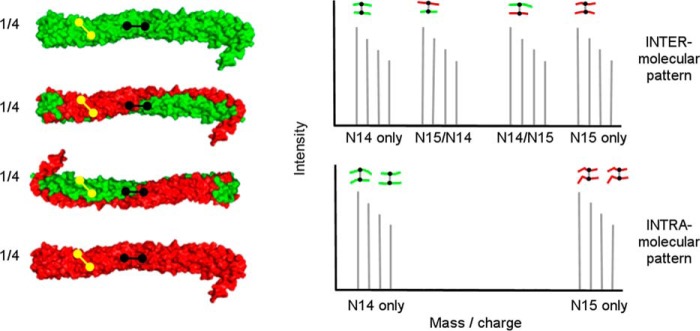
Principle behind isotope-assisted chemical cross-linking for determination of intra- versus intermolecular cross-links in oligomerized proteins. Recombinant proteins produced with either (i) 14N amino acids (green) or (ii) 15N amino acids (red) are thoroughly mixed at a 1:1 mass ratio under denaturing conditions and then allowed to reassociate, resulting in the four combinations shown on the left. The proteins are then cross-linked and fully digested with trypsin. For an intermolecular cross-link (shown in black) between two peptides (A and B), there are four possible mass combinations: 14A to 14B, 15A to 15B, and the mixed forms 14A to 15B and 14B to 15A. Thus, intermolecular cross-links will exhibit four mass peaks of approximately equal intensity as shown in the mass spectrum on the right. On the other hand, intramolecular cross-links (shown in yellow) only have two possibilities: 14A to 14B and 15A to 15B, allowing for an unambiguous determination of the cross-link molecular span.
