FIGURE 1.
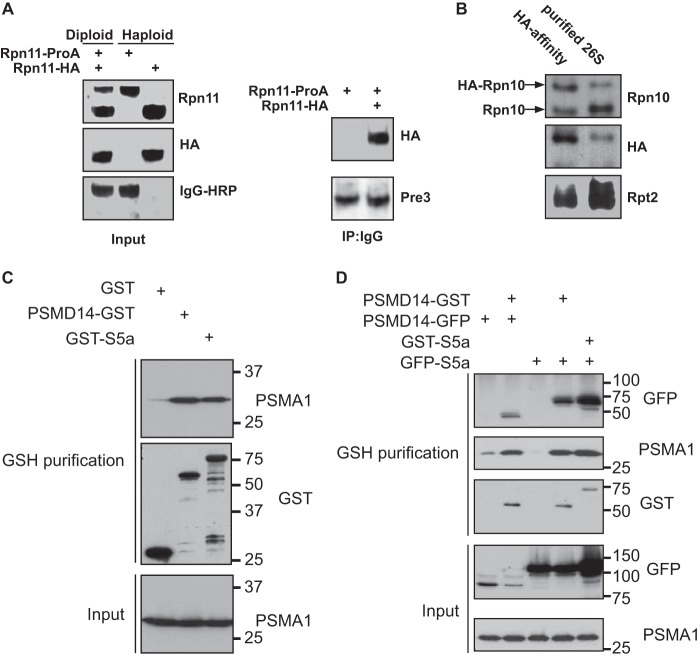
Rpn10 is present in the 19S complexes on both ends of a DC 26S proteasome. A, left panel: Western blot analysis of haploid strains expressing Rpn11-proA or Rpn11–3×HA, as well as diploid yeast expressing both differentially tagged versions of Rpn11. Right panel: 26S proteasome affinity purified on IgG beads were released from the matrix by TEV protease and analyzed for the present of HA-tagged Rpn11. Pulldown of complete proteasome were verified by immunodetection of a 20S subunit in the immunoprecipitate from both haploid and diploid strains. B, 26S proteasome were affinity purified using IgG-linked beads from a diploid strain expressing 3×HA-tagged and wild-type Rpn10 as well as Rpn11-ProA (Table 1). The 26S particles were released from the matrix by TEV protease and were then incubated with agarose beads decorated with HA antibodies. The immunoprecipitate was subjected to Western blot analysis using anti Rpn10, HA, and Rpt2 antibodies. Both wild-type and 3×HA-tagged Rpn10 were detected indicating that the two Rpn10 versions are present at least in some of the 26S proteasomes. Co-precipitation of other proteasomal subunits was confirmed by the detection of Rpt2. C, 293 cells were transfected with the indicated expression plasmids and subjected to glutathione affinity purification. Proteasomal purification by the GSH matrix was evaluated by immunoblots toward PSMA1. D, 293 cells were transfected with the indicated expression plasmids, and cell lysates were fractionated by velocity gradient centrifugation. Proteasome-containing fractions were pooled and subjected to glutathione affinity purification. Existence of doubly bound PSMD14 and S5a proteasome complexes was determined by a GFP immunoblot.