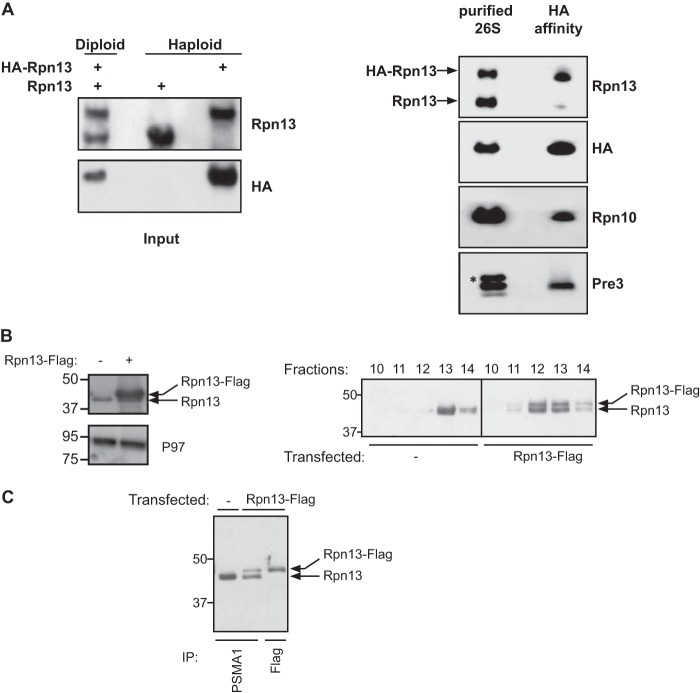
FIGURE 2.
A single Rpn13 subunit is present in DC 26S proteasome. A, left panel: Western blot analysis of total cell lysate and HA immunoprecipitate prepared from haploid yeast strains expressing a genomic version of wild type and 3×HA N-terminally tagged Rpn13, as well as a diploid strain expressing both versions of Rpn13. Right panel: 26S proteasome were affinity-purified using IgG-linked beads from a diploid strain expressing 3×HA-tagged and wild-type Rpn13 as well as Rpn11-ProA (Table 1). The 26S particles were released from the matrix by TEV protease and were then subjected to a HA affinity purification. Affinity-purified content was revealed using anti Rpn13, HA, Rpn10, and Pre3 antibodies. Only the 3×HA-tagged Rpn13 was detected, indicating presence of a single copy of Rpn13 in a 26S proteasomes. Co-precipitation of other proteasomal subunits was confirmed by the detection of Rpn10 and Pre3. An asterisk indicates a nonspecific band. B, cell lysates from the indicated cells directly evaluated toward the total Rpn13 and P97 content (left panel). The indicated lysates were fractionated on a glycerol gradient and split into 14 equal fractions and Rpn13 content in the HMW fractions was evaluated by an Rpn13 immunoblot detecting both the endogenous and exogenous Rpn13 forms (right panel). C, proteasomal fractions (fractions 10–14) from B were subjected to a PSMA1 or a FLAG affinity purification as indicated. Note that although the PSMA1 purification from the transfected cells had a higher endogenous Rpn13 content, this isoform was completely absent from the FLAG purification.