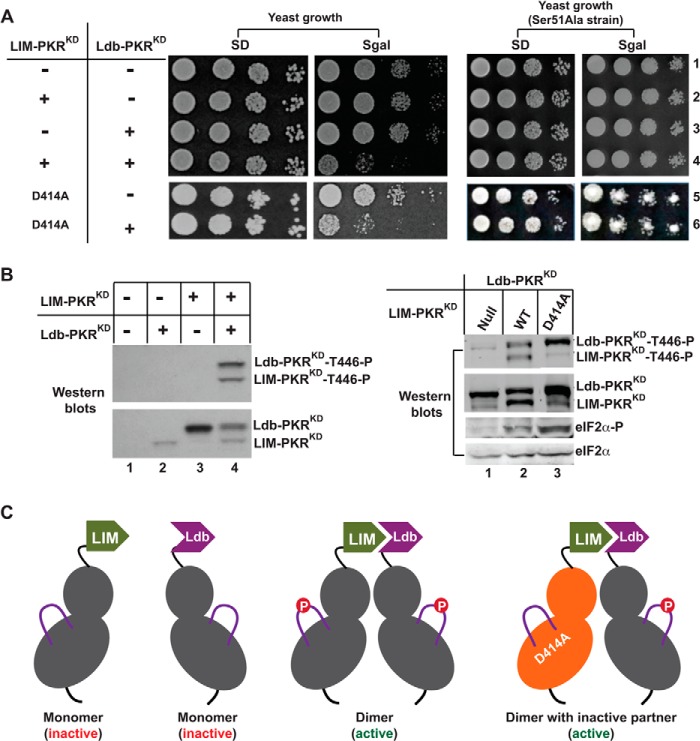
FIGURE 2.
Thr-446 phosphorylation occurs in a cis mechanism following dimerization. A, heterologous dimerization domains activate the PKR KD. The LIM-PKRKD fusion gene in the plasmid pC901 with a URA3 selectable marker and the Ldb-PKRKD fusion gene in the plasmid pC903 with a TRP selectable marker were introduced into WT yeast strain H2557 as well as S51A strain. The sign (+) means the presence, whereas the sign (−) means the absence of pC901/pC903 plasmids but contains the respective vector plasmid. The indicated D414A mutation was introduced in to the LIM-PKRKD fusion construct. Transformants were tested for growth on SD and SGal media. The PKR was inactive when the yeast grew on SGal medium, but active if the yeast did not grow. B, analysis of Thr-446 autophosphorylation and eIF2α phosphorylation. Yeast strains expressing indicated LIM- PKRKD (WT or D414A) and Ldb-PKRKD fusion protein were grown in the SGal medium and whole cell extracts were subjected to Western blot analysis using either a Thr-446-phosphospecific (T446∼P) or Ser-51 phosphospecific (eIF2α∼P) antibody followed by a polyclonal antibody of PKR or eIF2α. C, models represent mono- or dimeric form of LIM- and Ldb-PKRKD fusion proteins. The bi-lobal PKR-KD is shown in gray whereas the activation loops in purple solid lines with a phosphorylated residue (red circle). The inactive form of the kinase domain is colored orange.