FIGURE 5.
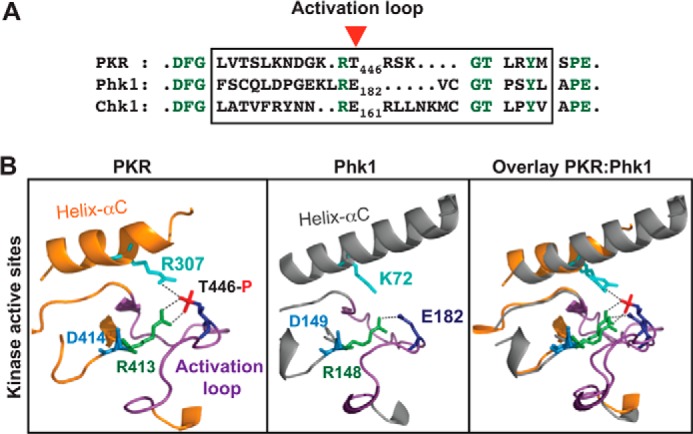
Kinase domains of PKR and Phk1 are structurally similar. A, residue E182 in Phk1 corresponds to the residue Thr-446 in PKR. The activation loop residues (boxed) of PKR, Phk1, and Chk1 were compared by pairwise alignment. The conserved residues are colored in green. A red arrowhead shows Thr-446 of PKR. B, kinase domains of PKR and Phk1 superimpose well on each other. Structural coordinates of the kinase domains of PKR (PDB ID: 2A1A) and Phk1 (PDB ID: 2PHK) were superimposed, using computer software PyMol. Proteins are shown as a ribbon presentation. For clarity, several structural elements are omitted. Only the helix-αC (colored orange in PKR whereas gray in Phk1) and the activation loop (colored purple both in PKR and Phk1) in the active site regions are shown. The conserved Arg-413 of the RD motif (R413 colored green) contacts with phosphorylated Thr-446 in PKR, whereas the corresponding Arg-148 in Phk1 (R148 colored green) contacts with phospho-mimetic E182. The catalytic base aspartate (D414 in PKR or D149 in Phk1) is colored in blue.
