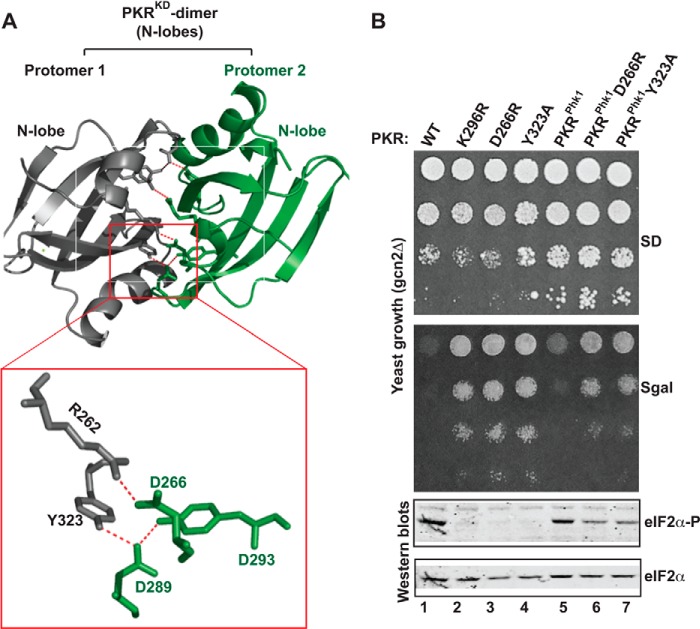
FIGURE 6.
Salt-bridge interactions at the dimer interface are partially important for PKRphk1 function. A, intra- and inter-molecular salt-bridge interactions at the PKR-KD dimer interface. A dimer of the PKR kinase domains (PDB ID: 2A19) is represented as a ribbon diagram. For clarity, only the interacting N-terminal lobes are shown. Several intra- and inter-molecular hydrophobic and salt-bridge interactions stabilize the dimer interface between protomer 1 (gray) and protomer 2 (green). Only two prominent salt-bridge interactions (R262·D266 and D289·Y293·Y323) are zoomed in a separate box. B, in vivo analysis of PKR mutants by growth in yeast and by eIF2α phosphorylation. The gcn2Δ yeast strains harboring indicated PKR mutants were tested for growth on SD and SGal media (upper panels). Whole cell extracts from these cells were subjected to Western blot analyses using antibodies of phosphorylated eIF2α (eIF2α∼P) and eIF2α.