FIGURE 7.
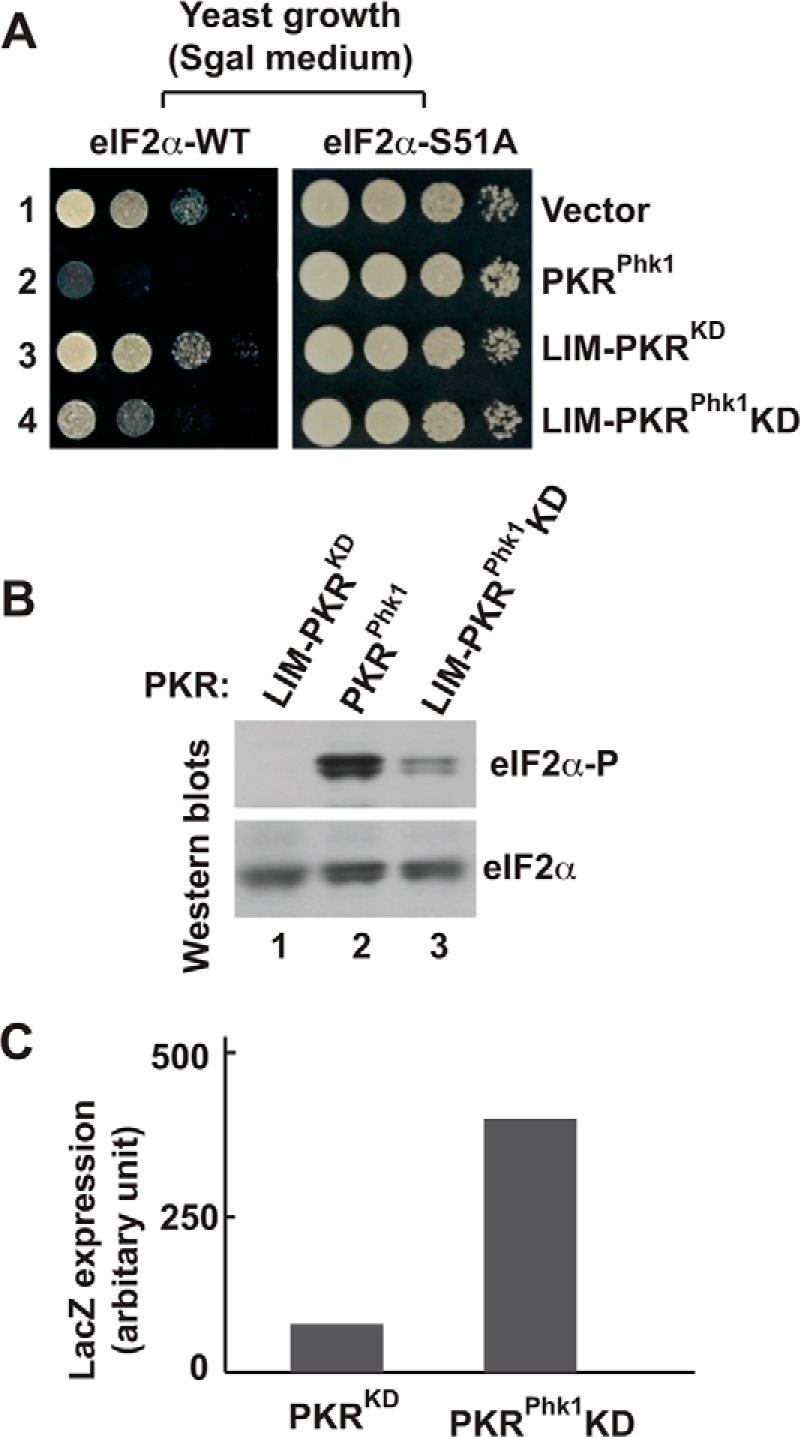
The kinase domain of PKRphk1 chimera is partially active. A, in vivo analysis of PKR mutants by growth in yeast. The WT (eIF2α-WT) and S51A (eIF2α-S51A) yeast strains harboring indicated PKR mutants were serially diluted and tested for growth on SGal medium. B, PKRKD-Phk1 phosphorylates eIF2α. Whole cell extracts from cells expressing indicated PKR derivatives were subjected to Western blot analyses using antibodies of phosphorylated eIF2α (eIF2α∼P) and eIF2α. C, PKRKD-Phk1 can activate the GCN4 translational control. Whole cell extracts of yeast cells carrying a GCN4 LacZ reporter plasmid p180 were prepared and β-gal activities were monitored as described previously (36).
