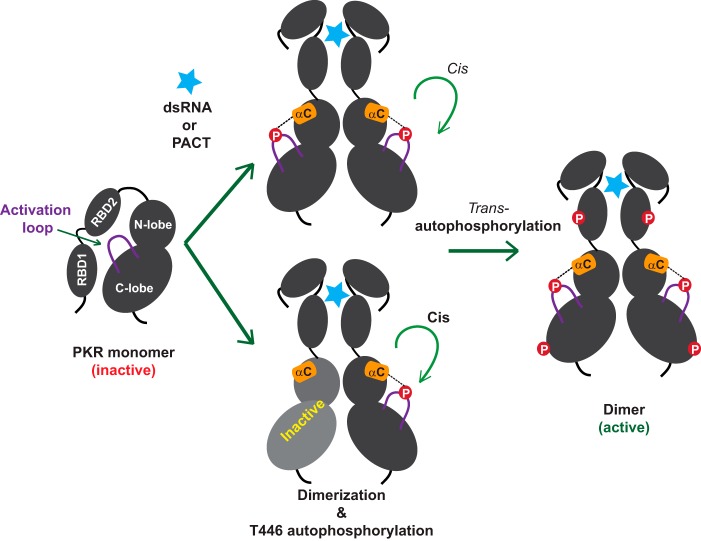
FIGURE 8.
Maturation and activation of PKR by autophosphorylation reactions. A conceptual model of the inactive PKR in which the RBD2 is bound to the kinase domain (40) and the activation loop (solid purple line) is un-phosphorylated. Binding of dsRNA or PACT (light blue star) to the RBD releases the autoinhibition (40). Consequently, the kinase domains of PKR adopt a favorable dimeric configuration (even with an inactive KD partner shown in gray) that facilitates autophosphorylation on the residue Thr-446 in cis. As shown by dotted lines, the phosphorylated Thr-446 couples with the helix-αC (orange cylinder) and an active closed conformation is achieved. Then PKR phosphorylates other residues in trans (shown in red circles) and becomes fully active.