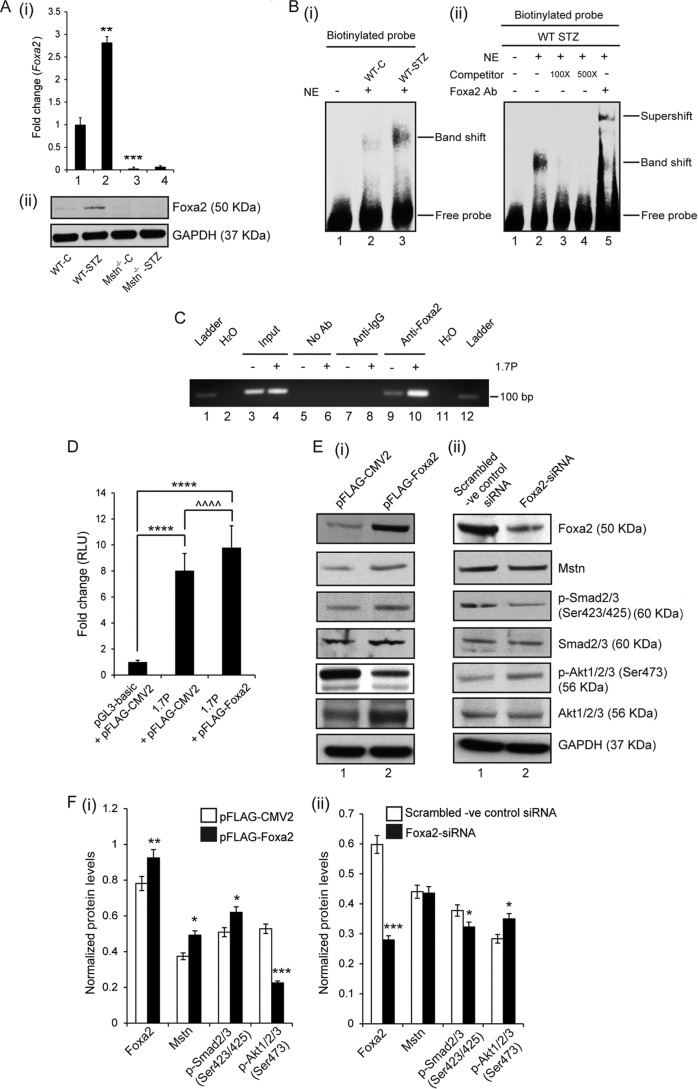
FIGURE 2.
Foxa2 mediated up-regulation of Mstn in response to STZ. A, representative graph (i) showing mRNA expression of Foxa2 and representative Western blot (ii) showing protein levels of Foxa2 in WT-C, WT-STZ, Mstn−/−-C and Mstn−/−-STZ muscle (**, p < 0.01, ***, p < 0.001 when compared with WT-C muscle, n = 7). B, (i) left panel, representative electrophoretic mobility shift assay gel showing increased Foxa2 binding to the DNA upon STZ treatment as indicated by the shifted band in lane 3 (lane 1-oligo only, lane 2-WT-C, lane 3-WT-STZ). (ii) Right panel, representative gel showing the disappearance of the shifted band when nuclear extracts of WT-STZ Biceps femoris muscles were incubated with increasing concentrations of competitor oligos (100× and 500×). Supershift of the Foxa2 specific band when WT-STZ Biceps femoris muscle nuclear extracts were pre-incubated with Foxa2 antibody (lane 1-oligo only, lane 2-WT-STZ, lane 3-with 100× competitor oligo, lane 4-with 500× competitor oligo, lane 5-with Foxa2 antibody) (n = 3). C, representative agarose gel image showing the binding of Foxa2 to 1.7 kb murine Mstn promoter (1.7P) (lanes 9 and 10), as assessed by ChIP. The relative amounts of both the control and Mstn promoter in the input were also assessed (lanes 3 and 4). Both no antibody (No Ab) (lanes 5 and 6) and isotype specific IgG (lanes 7 and 8) controls are shown. D, assessment of promoter-luciferase reporter activity, expressed as relative luminescence units (RLU) in C2C12 myoblasts transfected with either pGL3-basic and pFLAG-CMV2 empty vectors or 1.7 kb mouse Mstn promoter construct (1.7P) and pFLAG-CMV2 or 1.7P and Foxa2 expression vector (pFLAG-Foxa2), together with the control Renilla luciferase vector pRL-TK, (****, p < 0.0001, ^^^^, p < 0.0001; n = 3). E, Western blot and (F) densitometric analysis of Foxa2, Mstn, p-Smad2/3, Smad2/3, p-Akt1/2/3, and Akt1/2/3 in protein lysates obtained from proliferating C2C12 cells transfected with either (i) p-FLAG-CMV2 or p-FLAG-Foxa2 or (ii) scrambled -ve control siRNA or Foxa2-siRNA. GAPDH was used as an internal control for equal protein loading on the gel (*, p < 0.05; **, p < 0.01; ***, p < 0.001). (n = 3).