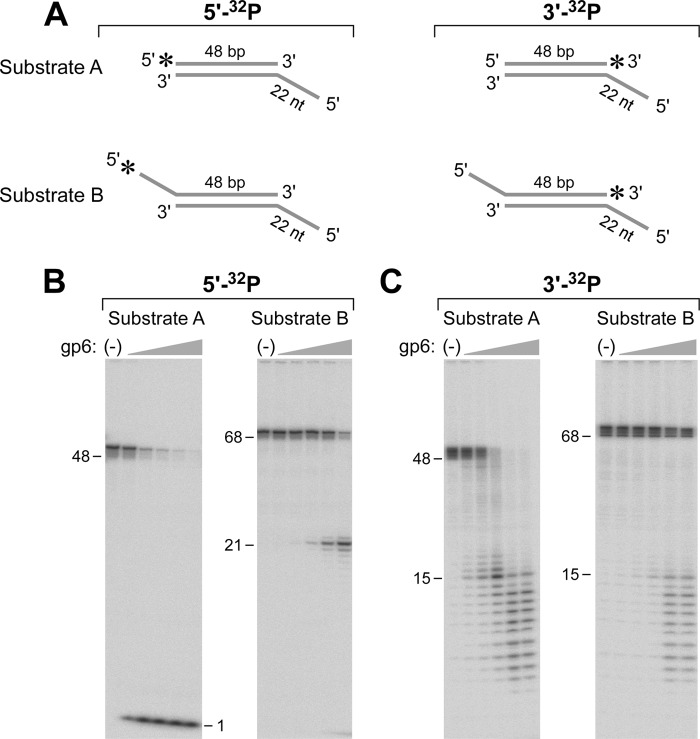
FIGURE 2.
gp6 removes a 5′-ssDNA tail on duplex DNA. A, Substrate A consists of a duplex of 48 bp with a 22-nt ssDNA tail attached to the 5′-terminus of one of the strands (A1:B1). The 48-nt strand was labeled with a 32P at either the 5′- or 3′-end. Substrate B is similar to Substrate A except that both ends have 5′-ssDNA tails, one with a 22-nt ssDNA tail and the other with a 20-nt tail. The strand bearing the 20-nt tail was labeled with a 32P at either the 5′- or 3′-terminus. The preparation of these substrates is described under “Experimental Procedures.” The 5′-overhang at the unlabeled end prevents rapid degradation of the duplex region from that terminus. B, Substrates A and B were labeled with 32P at their 5′-termini and incubated with gp6 as described under “Experimental Procedures.” Reactions (20 μl) were initiated by incubating increasing concentrations of gp6 (0.8, 4, 20, 100, and 500 nm) with a 50 nm concentration of the indicated DNA substrate. The radioactive oligoribonucleotide products were separated on an 18% polyacrylamide gel containing 7 m urea. The numbers on the left indicate the position and size of oligonucleotide standards. C, both Substrates A and B were labeled with 32P at their 3′- instead of their 5′-termini. Each substrate was incubated with gp6 and analyzed as described in B. The numbers on the left indicate the position and size of oligonucleotide standards.