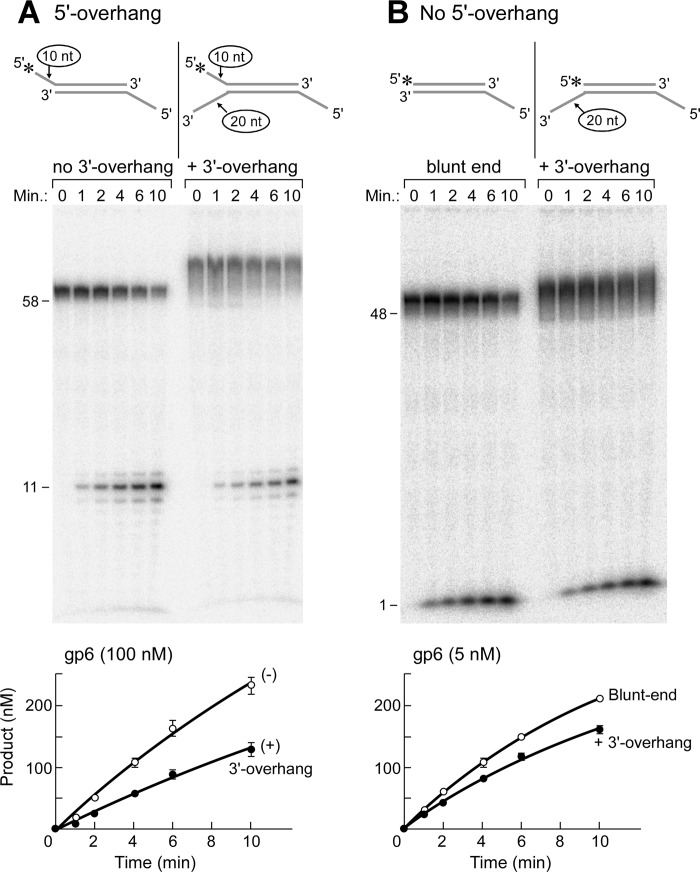
FIGURE 4.
Effect of 3′-extension near the cleavage site on flap endonuclease activity. A, a 3′-ssDNA extension of the complementary strand creates a Y-shaped structure (A4:B2). The efficiency of the flap endonuclease activity of gp6 was measured using a DNA substrate containing an extension (20 nt) of the 3′-end of the strand complementary to that bearing the 5′-flap. The 5′-terminus of the ssDNA extension of the 10-nt overhang was labeled with 32P, and a 22-nt 5′-overhang was present on the complementary strand to decrease the activity of the gp6 exonuclease activity on the 48-bp duplex region. A control DNA substrate lacks the 3′-ssDNA extension (A4:B1). Reactions were initiated by incubating gp6 (100 nm) with either substrate at 37 °C. The reaction products at the indicated time point were separated on a 20% polyacrylamide gel containing 7 m urea and analyzed using a PhosphorImager. The smearing observed above the uncut labeled strand (58 nt) is due to incomplete denaturation of DNA; the smear was considered as a non-cleaved substrate. The products of gp6 reactions containing the 10-nt 5′-overhang substrate with (closed circle) or without (open circle) the 3′-overhang were measured from three independent assays and are shown as plots of products versus time. Error bars, S.D. B, the same assay as in A was carried out except for using a substrate without a 5′-overhang (A1:B2) for measurement of the exonuclease activity. A blunt-ended substrate (A1:B1) served as a control. The products of gp6 reactions containing the 3′-extension substrate (closed circle) or the blunt-ended substrate (open circle) were measured from three independent assays and are shown as plots of products versus time. Error bars, S.D.