FIGURE 2.
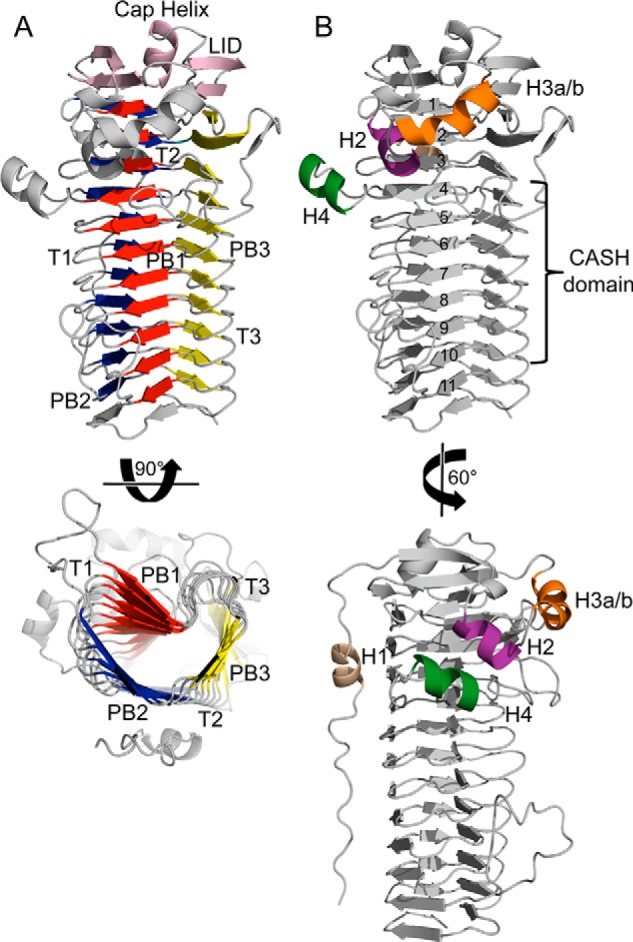
Overall structure of AlgG. A, schematic representation of AlgG. The parallel β-helix fold is made up of three parallel β-sheets, PB1 (red), PB2 (blue), and PB3 (yellow), which are connected via turns T1, T2, and T3. The parallel β-helix is capped N-terminally by an elaborate “lid” (pink). The lid consists of a short capping helix flanked by two antiparallel β-sheets. B, schematic representation of AlgG with coil numbers (1–11) and carbohydrate-binding/sugar hydrolysis (CASH) domain (22, 53). The N-terminal tail is packed against the PB2 surface and contains helix H1 (beige). AlgG contains three additional helices termed H2 (purple), H3a-H3b (orange), and H4 (dark green).
