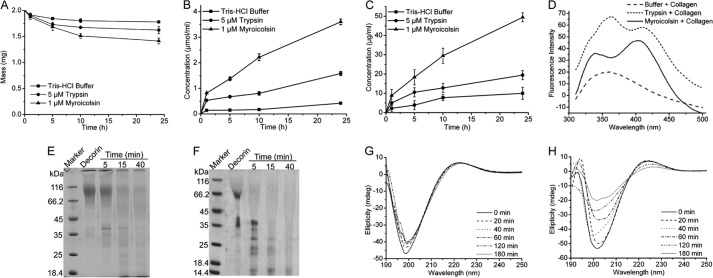
FIGURE 6.
The enzymatic degradation of the covalent cross-links in collagen fiber by myroicolsin. A, mass analysis of collagen fibers before and after incubation with 1 μm myroicolsin at 30 °C for 24 h. Collagen incubated with 5 μm trypsin or buffer B was used as a control. B and C, contents of amino acids (B) and GAGs (C) released from the degraded collagen. Collagen fibers (10 mg) were incubated with 1 μm myroicolsin, 5 μm trypsin, or buffer B at 30 °C for 24 h. The amino acid concentration in the supernatant of the digested mixture was quantitatively analyzed by the colorimetric ninhydrin method with l-leucine as the standard. The GAG concentration in the supernatant of the digested mixture was quantitatively analyzed by the dimethylmethylene blue assay. D, fluorescence spectra analysis of pyridinolines and deoxypyridinolines released from collagen telopeptides. Collagen fibers (20 mg) were incubated with 0.1 μm myroicolsin, 0.1 μm trypsin, or buffer B at 30 °C for 48 h. Pyridinolines and deoxypyridinolines in the supernatant of the digested mixture were detected on an FP-6500 spectrometer (Jasco, Japan) with an excitation wavelength of 295 nm at room temperature. E and F, SDS-PAGE analysis of decorin degradation by 0.5 μm trypsin (E) and 0.1 μm myroicolsin (F) at 30 °C. G and H, CD spectra of acid-soluble collagen incubated with 0.5 μm trypsin (G) and 0.1 μm myroicolsin (H) at 25 °C for 180 min. Error bars, S.D.