FIGURE 6.
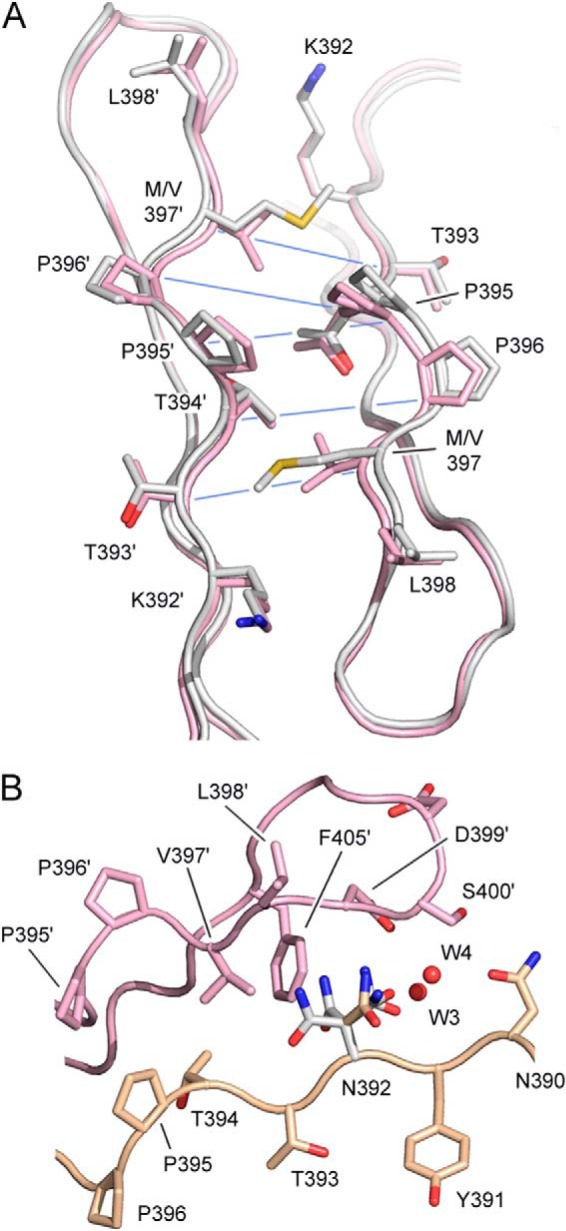
Effects of Met-397 and Asn-392 on the CH3-CH3 interface. A, the IgG1 (pink) and IgG2 (white) CH3-CH3 interface in the vicinity of residue 397, which is valine in IgG1 and methionine in IgG2. Cα-Cα distances, which are increased due to the presence of Met-397, are indicated with a blue line. PDB codes are 3AVE (51) and 4L4J (53), respectively. B, the IgG1 CH3-CH3 interface. Residue 392 is lysine in IgG1 but has been mutated to asparagine using an IgG1-Fc structure as a template (PDB code 3AVE). Asn-392 conformers colored in white are those that clash with Val-397′, Phe-405′, Asp-399′, and a conserved water molecule (W4). The conformer, colored in gold, is accommodated at the CH3-CH3 interface without any clashes and could form a hydrogen bond with Asp-399′ and surface water molecules (not shown). Carbon atoms from the two domains are colored in pink and gold.
