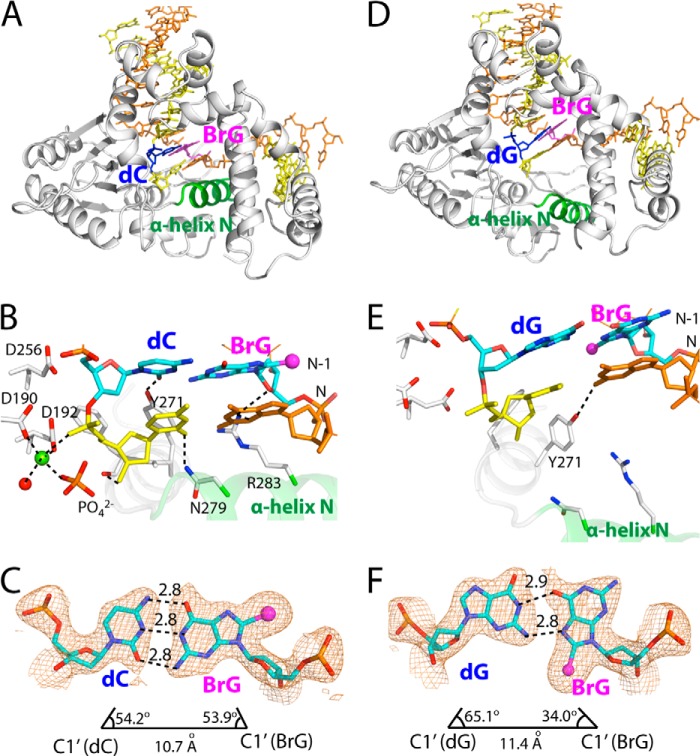
FIGURE 7.
Post-extension binary structures of polβ containing BrG·C or BrG·G at the N-1 position. A, overall structure of the BrG·C post-extension binary structure (PDB ID 4NM1). Protein is in closed conformation, and BrG·C forms co-planar base pair. B, active-site view of the BrG·C post-extension complex. Asn-279, Arg-283, and Tyr-271 are H-bonded to minor groove edges of bases. Note that a penta-coordinated Mg2+ (shown in yellow-green) is present near primer terminus C. C, H-bondings and geometry of BrG·C at the N-1 position. The λ angles and C1′-C1′ distance are similar to those for normal Watson-Crick base pair. A 2Fo − Fc map is contoured at 1σ around BrG and dC. D, overall structure of the BrG·G post-extension binary structure (PDB ID 4NM2). Protein is in open conformation, and BrG·G is in co-planar conformation. E, active-site view of the BrG·C post-extension complex. Asn-279 and Arg-283 do not engage in H-bonding interaction with the primer terminus base pair. Note that a metal ion is not present near primer terminus base. F, H-bondings and geometry of BrG·G at the N-1 position. The C1′-C1′ distance is longer than that for the BrG·C base pair.