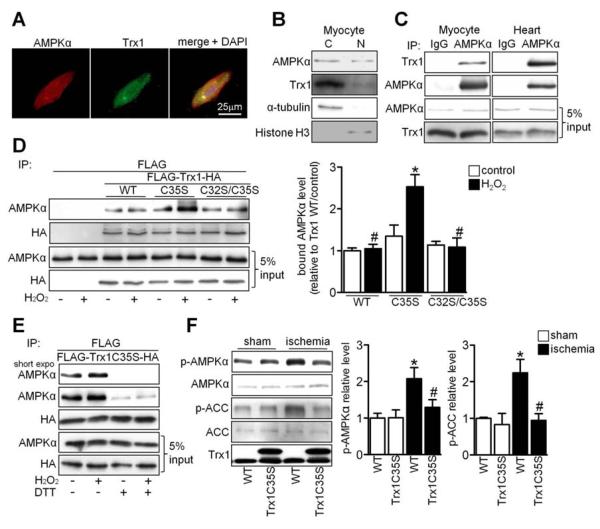
Figure 2. AMPK Interacts with the Active Site of Trx1.
(A) Myocytes were stained with AMPKα antibody (red), Trx1 antibody (green) and DAPI (blue). (B) The presence of AMPKα and Trx1 was examined in cytosolic and nuclear fractions prepared from neonatal cardiomyocytes. (C) Interaction between AMPKα and Trx1 was examined. Results are representative of three individual experiments. (D-E) Cos7 cells were transfected with N-terminal FLAG and C-terminal HA tagged Trx1 WT (FLAG-Trx1-HA) or mutant plasmids. (D) Cells transfected with the indicated plasmids were treated with or without 100 μM H2O2 for 30 min. Interaction between AMPKα and Trx1 mutants was examined. Statistical analysis of the immunoblot densitomeric measurements is shown (*p<0.05 vs. WT/H2O2, #p<0.05 vs. C35S/H2O2, n=3). (E) Cells transfected with the indicated plasmids were treated with or without 100 μM H2O2 for 30 min and lysed with or without 1 mM DTT. Immunoblots with AMPKα and HA antibodies are shown. (F) Tg-Trx1C35S and WT mice were subjected to ischemia for 20 min. Homogenates prepared from sham-operated hearts and the ischemic areas were used for immunoblot analyses of p-AMPKα, AMPKα, p-ACC, ACC, and Trx1. Statistical analyses of densitomeric measurements of p-AMPKα and p-ACC are shown (*p<0.05 vs. WT/sham, #p<0.05 vs. WT/ischemia, n=3-5). Error bars represent SEM.