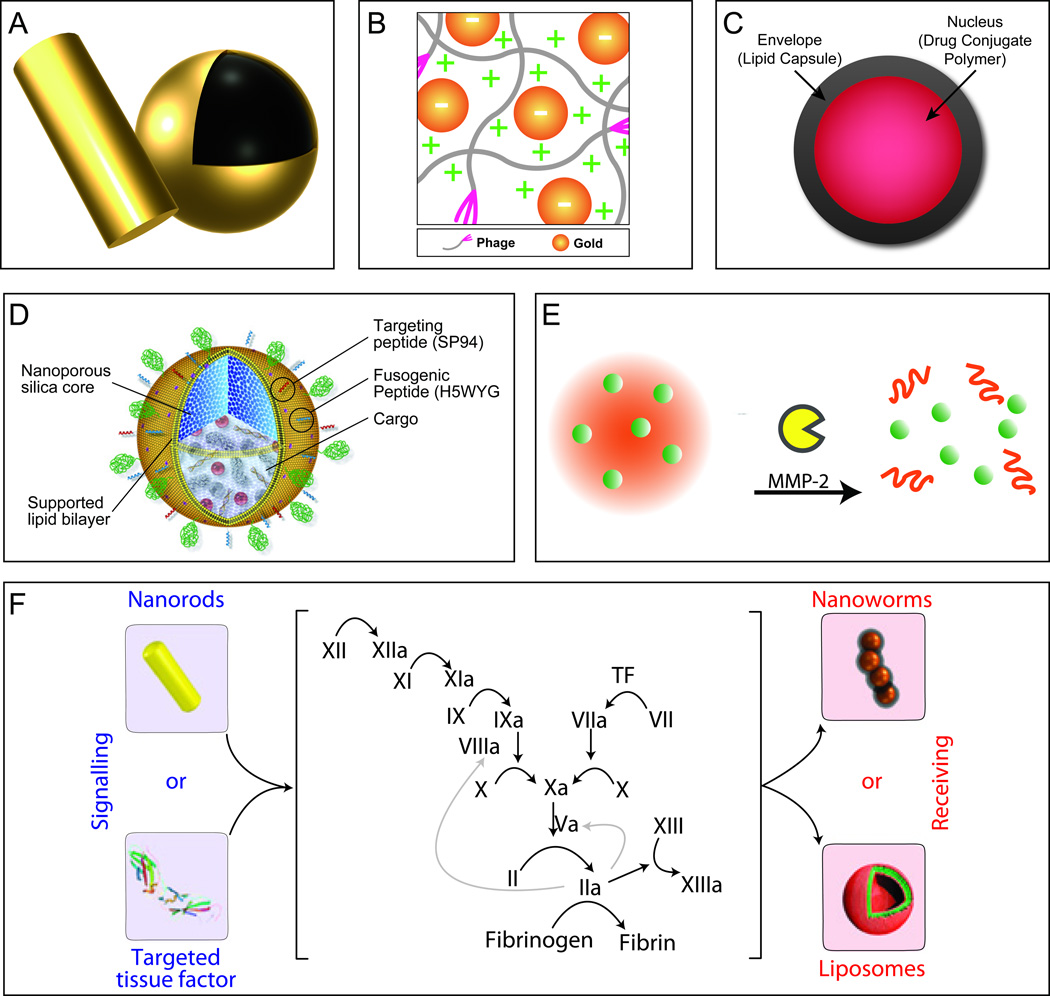
Figure 2. Third-generation nanocarrier platforms.
A. Gold nanoshells and rods display unique photothermal properties and can enhance fluorescent and magnetic properties when coupled to their surface. B. Au-phage networks are the result of the unique synergism yielded upon the incorporation of gold nanoparticles with bacteriophages, schematic is shown here displaying the effect of charge. Adapted from [41] and reproduced by permission from Jonathan O. Martinez, Methods in Bioengineering: Nanoscale Bioengineering and Nanomedicine, Norwood, MA: Artech House, Inc., 2009. © 2009 by Artech House, Inc. C. Nanocells are comprised of two compartments (nucleus and envelope) which allows for the temporal release of agents enabling time-dependent delivery of therapeutics, adapted from [43]. D. Protocells are nanoporous silica cores supported by a lipid bilayer. The porous core allows for the incorporation of a diverse array of cargoes, while the lipid bilayer enables for further conjugation of targeting and fusogenic peptides yielding an agent capable of providing a “one nanoparticle, one kill”. Reproduced with permission from NPG [44] E. Embedded nanoparticles enable the concentrated delivery of smaller nanoparticles deep within the tumor site by taking advantage of the responsive nature of larger nanoparticles to tumor microenvironment cues (e.g., Gelatin & MMP-2). Reproduced from [49] with permission from National Academy of Sciences, U.S.A. F. Communicating nanoparticles benefit from the amplification of signals created through biological cascades resulting in significant increases in nanoparticle tumor accumulation. Using the increases in factors involved in the coagulation cascade, via the heating of gold nanorods, one could effectively increase the accumulation of targeted therapeutic and diagnostic agents. Reproduced with permission from NPG [50].