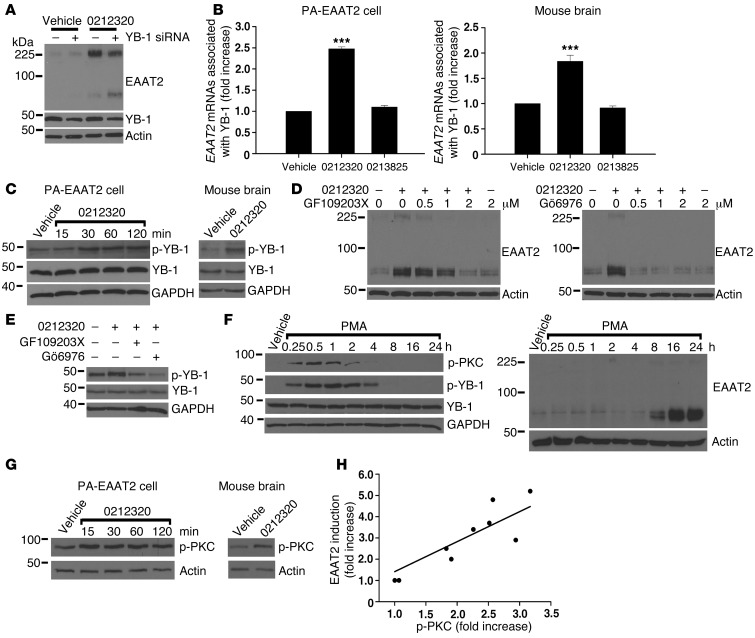
Figure 6. LDN/OSU-0212320 treatment results in activation of PKC, which subsequently activates YB-1, leading to enhancement of EAAT2 translation.
(A) Depletion of YB-1 by siRNA reduced compound-induced EAAT2 expression in PA-EAAT2 cells. Cells were transfected with YB-1 siRNA and were treated 24 hours later with compound (10 μM) for 24 hours. Lanes were run on the same gel but were noncontiguous. (B) Increased YB-1-EAAT2 mRNA interaction following compound treatment. PA-EAAT2 cells were treated with compound (10 μM) for 2 hours and then harvested for immunoprecipitation (anti–YB-1 antibodies). Coimmunoprecipitated EAAT2 mRNAs were quantified by real-time RT-PCR. Mice received a single dose of compound (40 mg/kg, i.p.), and brains were harvested at 2 hours for immunoprecipitation. n = 5. (C) Compound increased YB-1 phosphorylation (p-YB-1). PA-EAAT2 cells: 10 μM compound. Mouse brain: 40 mg/kg, harvested at 1 hour. (D) PKC inhibitors blocked compound-induced EAAT2 expression in PA-EAAT2 cells. Cells were pretreated with inhibitors for 1 hour and then treated with compound (10 μM) for 24 hours. (E) PKC inhibitors blocked compound-induced YB-1 phosphorylation in PA-EAAT2 cells. (F) PMA increased YB-1 phosphorylation concomitantly with PKC phosphorylation (p-PKC). Increased EAAT2 protein levels were not observed until 8 hours after treatment. (G) Compound increased PKC phosphorylation. PA-EAAT2 cells: 10 μM compound. Mouse brain: 40 mg/kg, harvested at 1 hour. (H) Compounds of different potencies were examined for their effects on PKC activation. p-PKC levels are highly correlated with EAAT2 induction levels. Each data point represents the mean of three experiments. ***P < 0.001.