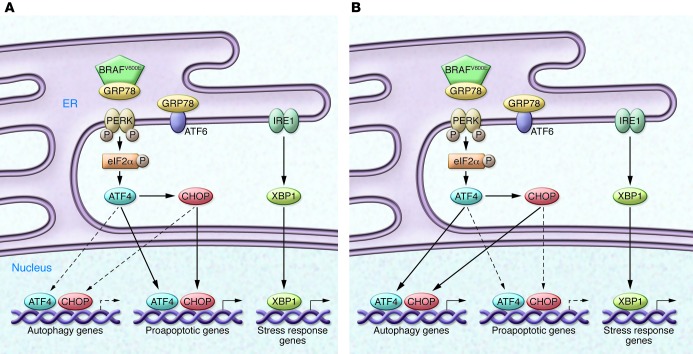
Figure 1. The BRAF inhibitor vemurafenib activates ER stress.
Vemurafenib promotes the association of BRAFV600E with GRP78 in the ER in drug-sensitive (A) and drug-resistant (B) melanoma cells. This association displaces GRP78 from PERK, resulting in robust autophosphorylation and kinase activation. The ensuing PERK-mediated phosphorylation of eIF2α initiates a transcriptional and translational cascade that is mediated by expression of the transcription factors ATF4 and CHOP. These nuclear factors either act alone or together to promote the expression of numerous proapoptotic — and, paradoxically, also autophagic (11) — genes. Vemurafenib treatment also results in IRE1 activation and subsequent splicing of the mRNA encoding X-box binding protein 1 (XBP1), a transcription factor that regulates ER-associated protein degradation in response to ER stress. In drug-sensitive melanomas (A), apoptosis prevails, while in drug-resistant tumor cells (B), autophagy may override apoptosis. The factors that shift the balance from apoptosis to autophagy in response to vemurafenib treatment remain unknown, but could include the expression of cellular IAPs or expression of other antiapoptotic genes that contribute to tumor survival in the presence of the BRAF inhibitor. The present findings of Ma et al. (8) suggest that autophagy inhibitors combined with vemurafenib may be an effective therapy for melanoma that would eliminate both drug-sensitive and drug-resistant tumor cells.