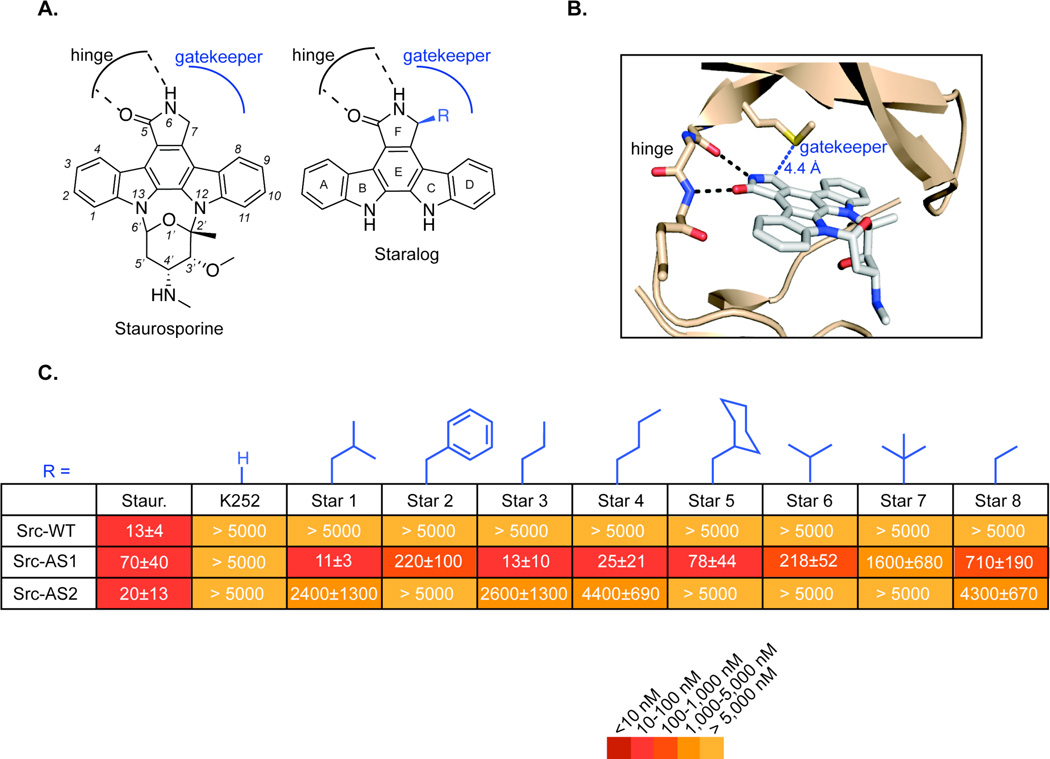
Figure 1.
Design of staralog inhibitors and structure-activity relationships of the C7-position. (A) Structure of staurosporine (left) and engineered staralog inhibitors (right) with kinase hinge-binding interaction and gatekeeper pocket illustrated in black and blue, respectively. (B) X-ray co-crystal structure of staurosporine bound to Protein Kinase Cθ14 with hinge interaction highlighted in black and the distance between C7-position of staurosporine and the Met gatekeeper side chain highlighted in blue. (C) IC50 values for staralog inhibitors against Src-WT, Src-AS1, and Src-AS2 kinases. IC50 values were determined from a seven-point dose-response. Each experiment was done in triplicate and repeated for a minimum total of three times for compounds that showed measureable inhibition. Compounds that showed no inhibition were measured twice. Reported values represent the mean and error represents the standard error of the mean. WT = native gatekeeper (Thr for Src), AS1 = Gly gatekeeper, AS2 = Ala gatekeeper