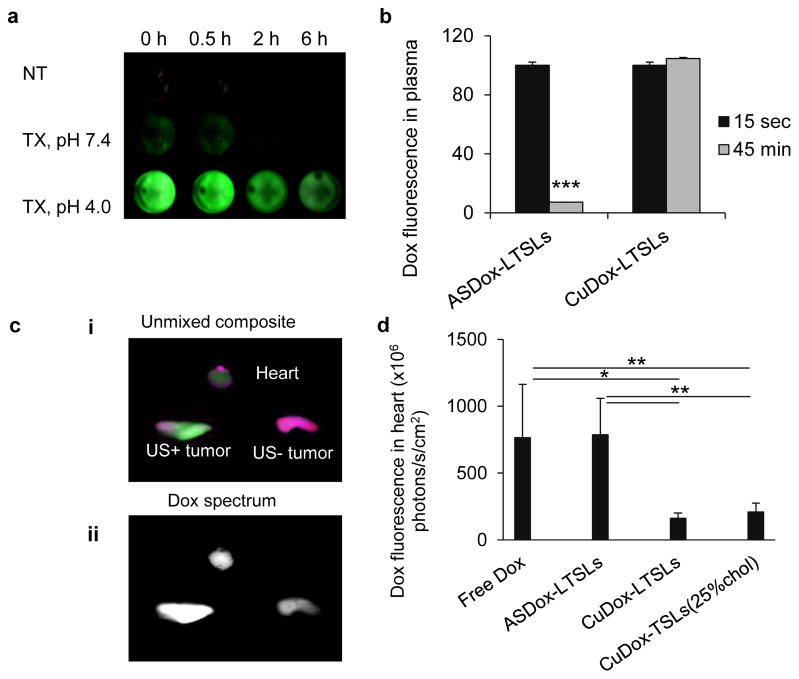
Figure 3. Blood stability of CuDox-LTSLs.
a) Dox fluorescence in plasma isolated from mice at 0, 0.5, 2 and 6 h post injection of CuDox-LTSLs and incubated for 30 min with either 20 mM HEPES/150 mM sodium chloride at pH 7.4 and room temperature (NT), 0.25% Triton X-100 in 20 mM HEPES/150 mM sodium chloride, pH 7.4, at 37 °C (TX, pH 7.4), or 20 mM citrate buffer/150 mM sodium chloride, pH 4.0, at 42 °C (TX, pH 4.0). Green indicates Dox fluorescence. b) Dox fluorescence intensity in plasma isolated from mouse blood after 15 sec and 45 min post injection of either ASDox-LTSLs or CuDox-LTSLs at a Dox concentration of 6 mg/kg body weight. c) Ex-vivo images of the heart and tumors of a mouse treated with ASDox-LTSLs combined with insonation of the left tumor at 42 °C for 20 min post drug injection. Images were acquired immediately after ultrasound and ~30 min post drug administration and are presented as an unmixed composite (i) of doxorubicin fluorescence in green and background in purple and (ii) as the Dox spectrum alone showing Dox fluorescence intensity (white indicates higher fluorescence intensity). d) Dox accumulation in the mouse heart quantified 30 min post administration of free Dox, ASDox-LTSLs, CuDox-LTSLs, and CuDox-TSLs. Statistical analyses were performed using Student’s t-test (b) and one-way ANOVA followed by the Tukey Post Hoc test (d). *p < 0.05, **p < 0.01, ***p<0.001.