Abstract
Chemokines produced in distinct tissue microenvironments sustain migration of mature lymphocytes in lymphoglandula. Chemokine receptors expressed on chronic lymphocytic leukemia (CLL) cells regulate the migration of the leukemia cells within the bone marrow (BM), lymphoid organs in collaboration with chemokines. Chemokines form a pro-survival circuitry by regulating leukocyte trafficking, maintaining extended lymphocyte survival. Therefore, chemokines in tumor cell–microenvironment interactions represent a target for treatment of CLL. AMD3100 disrupts the CLL/microenvironment interactions and influences CXCL12/CXCR4 survival signaling. Fostamatinib, ibrutinib, and GS-1101 as B-cell receptor (BCR)-related kinase inhibitors inhibit BCR- and chemokine-receptor-signal-regulated kinase and have a good clinical response in CLL. Lenalidomide, sorafenib, and dasatinib are other additional drugs associated with chemokine in microenvironment. Inhibiting signaling through chemokine and microenvironment associated signaling are emerging as innovative therapeutic targets in CLL. In this article, we reviewed the role of chemokines in CLL microenvironment and novel therapeutics targeting CLL microenvironment.
Keywords: chronic leukemia lymphoma, microenvironment, chemokines, chemokines receptors, targeted therapy
Introduction
Chronic lymphocytic leukemia (CLL) is considered as the accumulation of mature monoclonal B cells rather than proliferation indolent B cell characterized by defective apoptosis.1 Single gene mutations are rapidly being uncovered by sequencing the coding genome of CLL cases, including NOTCH1, splicing factor 3b subunit 1 (SF3B1), myeloid differentiation primary response gene 88 (MYD88).2 There is significant heterogeneity in the disease progression between CLL patients. Coding unmutated immunoglobulin variable heavy-chain (IGHV) genes and expressing the protein tyrosine kinase ZAP-70 and the type II transmembrane glycoprotein CD38 predict poor prognosis among leukemia patients who develop aggressive disease and need immediate therapy.1,3
Compared with normal lymphocytes, CLL cells are accumulated in the bone marrow (BM), lymphoid tissues, and are flowed into peripheral blood and prolong survival time in vivo. CLL cells are spontaneous apoptosis in vitro but can be rescued by microenvironment of BM and lymphoid tissues.4,5 CLL cells home to the BM by chemotaxis, increasing cell survival and probably the extent of marrow infiltration.6 In vitro, adding stromal cells promotes survival of CLL cells through the secretion of several soluble growth factors and proteins.7,8 CLL-accessory cell direct cross-talk in microenvironment appears to be meaningful in CLL cells survival and disease progression.9 The microenvironment in the BM and lymph nodes (LNs) provides drug-resistance signals for CLL cells and drug resistance mechanism can interpret minimal residual disease (MRD) after conventional treatments.10,11 Stromal cells protect CLL cells from conventional drug-induced apoptosis through cell adhesion-mediated drug resistance.
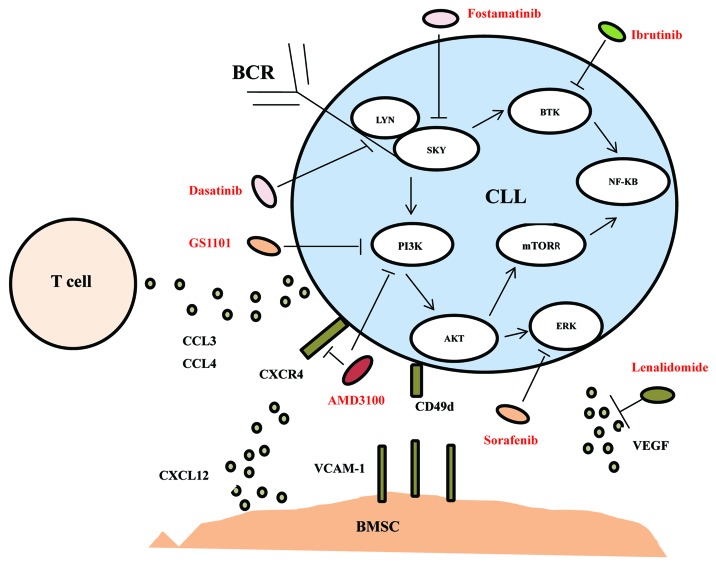
We will review the relationship of chemokines/chemokines receptors and CLL in microenvironment and then discuss therapeutic approaches of targeting the microenvironment or microenvironment associated signaling, as showed in Figure 1.
Figure 1. A schematic drawing of the microenvironmental interactions among CLL cells, T cells, and stromal cells described in the current review with targeting drugs.
Role of Chemokines in CLL Microenvironment
Chemokines as a family of approximately 50 peptides are first proposed as “chemotactic cytokines” in 1992 which play a role in regulating homing of immune cells, leukocyte trafficking and maturation.12,13 Physical interactions between CLL cells and bone marrow mesenchymal stem cells (BMSC), nurse-like cells (NLCs) are mediated through the molecular interaction of vascular cell adhesion molecule (VCAM-1), CD11a (leukocyte function associated antigen-1), and CD49d (very late antigen-4) and so on. Contact between the neoplastic cells and stroma-derived cells supports CLL cells growth and survival in vitro and in vivo.14 Topical study show long-term survival demands direct interaction between CLL cells and the stroma cell co-cultures, whereas short-term survival of CLL cells in vitro can be sustained by soluble factors produced by stromal cells.15 Stromal cell-derived factor-1 (SDF-1) as a homeostatic chemokine, binding to chemoreceptor CXCR4 not only plays a role in homing of CLL cells into the BM but also prolonging CLL cells survival by cell-to-cell interaction with BMSCs and NLCs.16 In CLL cells, homeostatic chemokine receptors CXCR5 and CCR7 lead in resistance-mediated apoptosis.17 The CX3CR1/CX3CL1 system may play a role in interactions between CLL cells and microenvironment by studying CXCL12- mediated adherence of leukemic cells to NLCs.18 Other chemokines like CLL-generated CCL3 and CCL4 significantly lead to the recruitment of cells from the monocyte/macrophage lineage to BM microenvironmental sites.19 Chemokines appear to form a pro-survival circuitry by regulating leukocyte trafficking, maintaining extended lymphocyte survival.20
Novel Therapeutics Targeting CLL Microenvironment
CXCR4 antagonists
CXCR4 (CD184), as a receptor for SDF-1(CXCL12) is highly expressed on the membrane of peripheral blood CLL cells which take advantage of CXCR4/CXCL12 axis to remain in a favorable environment.21 CXCL12 binding to CXCR4 can regulate leukemia cells adhesion to actin polymerization, vascular endothelium and accommodate migration beneath and underneath BMSCs.22 In CLL cells, higher levels of CD49d in fact conduct migration beneath BMSCs in assistance with CXCR4.23 CD38+ CLL cells show higher levels of chemotaxis compared with CD38− CLL cells and activation of CD38+ CLL cells with a monoclonal antibody (mAb) enhanced CXCR4 chemotaxis toward CXCL12, and a blocking anti-CD38 mAb can inhibit this chemotaxis.24 Migration and survival in response to CXCL12 are associated with ZAP-70 expression, which is stimulated by B-cell receptor (BCR) signaling.25 When engaging in adhesion to stromal cells, CLL cells are resistant to the cytotoxic effects of common drugs in CLL patients, like corticosteroids and fludarabine.26 This adhesion-mediated drug resistance mechanism may explain MRD in the marrow and relapse found in CLL patients.27
CXCR4 antagonists were initially identified for HIV treatment and then found to induce leukocytosis, and recently are applied clinically for hematopoietic progenitors mobilization in lymphoma patients.28 CXCR4 antagonists, such as AMD3100, T140, and ALX40-4C, can block CLL–stroma interactions and then mobilize CLL cells from their protective microenvironments to the blood, becoming accessible to conventional drugs.29,30 Namely, AMD3100 not only inhibits CXCL12-mediated guanosine diphosphate (GDP) binding, free of calcium, chemotaxis but also disrupts the cell/MSCs or NLCs-based microenvironment interactions, blocks survival stimuli, and influences the survival signal provided by CXCL12.30,31 In CLL, mobilization and sensitization of leukemia cells could be achieved via combining a CXCR4 antagonist with conventional cytotoxic agents like fludarabine, cyclophosphamide, or established CLL drugs, like antibodies (rituximab or alemtuzumab), or combined immunochemotherapy.31-33
Andritsos et al.34 went on a study of the maximum tolerated dose of AMD3100 in combination with rituximab in relapsed CLL patients and the results showed that of 14 estimable patients, 5 (36%) had partial response (PR), 3 (21%) had stable disease for ≥ 2 mo and 6 (43%) had progressive disease (PD). On day 8, there was a median 3.8-fold increase in peripheral blood CLL cells, demonstrating CLL cells mobilization. On day 26 fewer peripheral blood CLL cells were found with a median fold increase of 1.5-fold. Under certain circumstance, maximum responses were detected in several months after completion treatment of rituximab. From above data, we can find AMD3100 in combination with rituximab is a new therapeutic avenue for relapsed CLL patients.
The underway CLL trial combines plerixafor with rituximab, and original data demonstate a plerixafor dose-dependent CLL-cell mobilization to the peripheral blood from tissue sanctuaries and indicate the safety of combination of plerixafor with rituximab. Future studies in CLL may combine a CXCR4 antagonist with established convention agents or antibodies so as to help to mobilize and eliminate residual CLL cells.
BCR-related kinase inhibitors
BCR stimulation signal plays a significant role in the occurrence and prognosis of CLL.35 First of all, disease progress of CLL patients are closely related with BCR variable area mutations; second, CLL cells restrictively express IgVH sequence BCR; third, no mutation of Ig and/or ZAP-70+ has priority response to stimulation of BCR.36,37 BCR launches a signaling cascade leading in expansion of CLL clone in company with other signals, like CD40 ligand, B-cell activating factors (BAFF), a proliferation-inducing ligand (APRIL), and so on.38 In CLL, targeting different components of the BCR pathway can accomplish through kinds of constitutively active pathways including spleen tyrosine kinase (Syk), bruton tyrosine kinase (Btk), and phosphatidylinositol 3-kinases (PI3K).39 Inhibition of both Syk and the PI3K pathway block the cross-talk between CLL cells with the microenvironment and what is more, inhibition of Btk, Syk, and PI3K would promote pro-apoptotic signals.40
Syk inhibitors (fostamatinib disodium), Btk inhibitors (ibrutinib), and PI3K inhibitors (GS-1101), which are BCR-related kinase inhibitors, have common characteristics in CLL treatment that these drugs can make LNs shrinkage and lymphocytes transitional increase in the first weeks of treatment because of CLL cells mobilization to peripheral blood from tissues.41-43 Interference of BCR signal not only affects related survival pathways, but also influences tissue homing and CLL cells residual. BCR-related kinase inhibitors inhibit CLL cells chemokine-mediated adhesion in response to CXCL12 or CXCL13 and migration beneath stromal cells. These drugs also can downregulate secretion of BCR-dependent chemokines (CCL3, CCL4) produced by CLL cells. They inhibit BCR- and chemokine-receptor-induced Akt and extracellular signal-regulated kinase (ERK 1/2) activation and then markedly inhibit CLL cells survival and migration.44-46
PI3Ks inhibitor
PI3Ks as mediating signals of cell surface receptors enzymes has four class I PI3K isozymes (PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ) accommodating different cellular functions by the production of phosphatidylinositol-3,4,5-triphosphate.47 Generation of phosphatidylinositol-3,4,5-triphosphate activates the downstream Akt, and the mammalian target of rapamycin (mTOR), which both have positive effects on cell survival, proliferation, and growth.48 Of the all PI3K isoforms, PI3Kδ has been shown to play a significant role in homeostasis and function in response to chemokines. GS-110 1 is an up-to-date PI3Kδ specific inhibitor that promotes CLL apoptosis, migration, homing.43 Akt activation is suppressed by GS-1101 by means of CD40-, TNFα-, fibronectin-, and BCR-derived PI3K signaling.49
Overall response rate (ORR) of single agent GS-1101 treatment in relapsed or refractory indolent CLL patients is 33%. Ninety-one percent of CLL patients treated with GS-1101 lead to a greater than 50% decrease in their lymph node disease.50 Recently, GS-1101-based combination therapies are in clinical trial and Coutre et al.51 launched a phase I study that showed overall ORR for the GS-1101/rituximab (R), GS-1101/bendamustine (B), and GS-1101/BR respectively were 78%, 82%, and 87%, and 1-y progression-free survival (PFS) rates were 74%, 88%, and 87% respectively. Base on GS-1101 data on the American Society of Hematology Congress, we find that GS-1101 and GS-1101-based combination therapies are becoming a valid therapeutic target in relapsed or refractory CLL patients.
BTK inhibitor
BTK as a member of the Tec family kinases is activated upstream by Src-family kinases and leads to downstream activation of essential cell survival pathways such as nuclear factor-κB (NFκB) and mitogen activated protein-kinase (MAPK).52 Mouse genetic ablation studies show that other BCR-pathway kinases rather than Btk have pleiotropic effects on kinds of cells; moreover BTK mutations in humans result in X-linked agammaglobulinemia (XLA), which is designated as a severe B cell-specific defects inherited disorder.53 Based on these evidences, we suppose Btk is a distinctively attractive kinase target for selective B-cell inhibition.
Research finds that the mouse B cells deficient in function of cytoplasmic tyrosine kinase Btk are also lack of CXCL12-CXCR4, CXCL13-CXCR5, VCAM-1, or α4 integrin.54 Combining this notion with the recent finding, we presume that Btk may be involved in the signaling mechanism underlying chemokine-controlled integrin-mediated migration. It is becoming clear that Btk signaling downstream other receptors including CXCR4 and CXCR5 greatly influence the CLL hazard rank and disease progression.55 Ibrutinib is the first human Btk inhibitor binding particularly and irreversibly to Btk protein through a cysteine residue and then inhibiting Btk phosphorylation. Hoellenreiger et al.56 evaluate the effect of ibrutinib on CLL cell viability after anti-IgM stimulation and find ibrutinib blocks BCR-triggered CLL cells survival but CLL cells from ibrutinib-treated patients still are anti-IgM. Btk-independent pro-survival effects could not be inhibited by ibrutinib in vitro. Shortly after ibrutinib treatment, most circulating CLL cells display low CXCR4 expression which is characteristic of LN- and BM-derived CLL cells.
Brown et al.57 conduct the major clinical trials of BTK inhibitor. The result shows the ORR of ibrutinib in relapsed refractory CLL is 67% and PFS 88% at 15 mo. In a cohort of untreated patients 65 y and over, the estimated 15 mo PFS is 96%. ORR of combination of ibrutinib with BR is 93%, PFS 90% at 11 mo, compared with ibrutinib with ofatumumab, ORR 100%, PFS 89% at 10 mo. Burger and his colleagues conduct a phase II single-center clinical trials of ibrutinib and rituximab. Early evaluable response is 50% at the inital 3 mo; ORR is 85%, CR 40%, and PR 45%. On this combination trial, in contrast with single-agent ibrutinib, re-distribution lymphocytosis peaks earlier and the duration is shorter, presumably because of the addition of rituximab.58 On the basis of above data, ibrutinib and ibrutinib-based combination therapies is a safe, well-tolerated treatment for high-risk CLL patients and induce very high initial response rates.
Syk inhibitor
Syk as a member of the Syk/ZAP-70 family of non-receptor kinases activates BCR downstream signaling pathways, like Btk and activated B-cell linker protein (BLNK), which then activate the downstream signaling molecules NFκB, Raf, MEK, and ERK.59 Syk signaling is required for B-cell development, proliferation, and survival. Syk-deficient mice show an interdict at the pro-B to pre-B transition.60 R406 as an ATP competitive kinase inhibitor has limited specificity toward Syk, because of its activity against other kinases including FMS-related tyrosine kinases 3 (Flt3), Janus kinase 1, and Janus kinase 3. R406 is effective in CLL and other B-cell malignancies through disrupting BCR signals and micro-environmental interactions.61
Herman et al.62 research shows that NFκB signature genes and MYC signature genes are downregulated due to fostamatinib. Expression of CD69, CD86, and the percentage of CLL cells expressing Ki67 are also remarkably reduced by fostamatinib. Cytotoxic effects of Syk inhibitor is associated with Syk protein expression and is stronger in unmutated IGVH and ZAP70+ CLL cases. Compared with fludarabine therapy alone, combination of fludarabine with R406 increase cytotoxicity that provides potential mechanistic for a novel treatment option for the poor prognosis of CLL patients. Friedberg et al.63 launch a clinical trial, 68 patients are enrolled in three cohorts: diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), and other non-Hodgkin lymphoma (NHL). CLL/small lymphoma leukemia (SLL) has the highest RR that is 55% (6/11) to fostamatinib. Collectively the data provide a blueprint to further study fostamatinib-targeted therapeutics.
In these trials, we find BCR-related kinase inhibitors both can make LNs shrinkage and lymphocytes transitional increase in the initial weeks of treatment owing to CLL cells mobilization to blood. Data regard to the frequency of relapses and progression of BCR-related kinase inhibitors are at the present stage very juvenile, but preliminary results are satisfactory and myelosuppression is scarce. Now novel insights regarding BCR inhibitor drugs not only provide support for their further application as monotherapy but also for their use as equitable combination therapy like coalescence with rituximab, ofatumumab, and bendamustine, utilizing the microenvironment dependence in CLL. Potential mechanism of resistance to BCR inhibitor drugs as yet are unknown and probably will become an innovative avenue in future, especially when these drugs are widely application.
Lenalidomide
Lenalidomide as an immune modulatory agent are recently approved for application in multiple myeloma, lymphoma, acute myeloid leukemia and CLL.64 Application of lenalidomide in CLL has been combined with development of antitumor antibodies and induce a disease-specific side effect of tumor flare and cytokine release, such as serum basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF).65,66 bFGF and VEGF both have been associated with promoting CLL survival and circulating levels of bFGF is associated with response to therapy for CLL.65 Recent study shows that activation of CLL cells induced by lenalidomide depends on the PI3Kδ pathway. Inhibition of PI3Kδ signaling by the PI3Kδ inhibitor can block up CLL cell activation, VEGF and bFGF gene expression induced by lenalidomide.67
Now, the encouraging results with single-agent lenalidomide as first-line treatment option for CLL shows ORR is 56% (no CR) and tumor flare is common.68 Combination lenalidomide with rituximab of phase II clinical trials are ongoing in untreated CLL patients. The data present ORR is 66% and low toxicity have been observed.69 In vitro, combination of PI3Kδ inhibitor is effective for preventing the tumor flare produced by lenalidomide treatment. The evidence provides support for the combination of lenalidomide with PI3K inhibitors, and potentially other BCR signaling inhibitors as a novel chemoimmunotherapy in future.
Sorafenib
The multikinase inhibitor sorafenib as a promising agent for treating tumors is a small molecule inhibitor of RAF.70 Sorafenib could be particularly relevant in CLL cells by blocking CXCL12-induced phosphorylation of ERK and MEK in ZAP-70+ CLL cells. What is more, ZAP-70+ CLL cells represent more sensitive to the cytotoxic effects of sorafenib in vitro compared with ZAP-70− CLL cells.71 This agent could overcome the protective effect of the CLL microenvironment at different ranks, like prosurvival signaling, chemokine signaling. Thus, further discussion of these factors and their effects on CLL provide vast ground for the development of additional strategies to improve the effectiveness of treatment with high risk CLL patients.
Dasatinib
Dasatinib as a tyrosine kinase inhibitor is a “second-generation” ATP-competitive inhibitor of the oncogenic BCR-ABL kinase.72 Given dasatinib inhibiting all Src-family tyrosine kinases, research shows dasatinib can inhibit BCR signal transduction and furthermore block BCR-mediated survival of CLL cells.73 Dasatinib also significantly interferes migration of CLL cells toward CXCL12 through inhibiting CXCR4 signaling.74 There is only one published phase II trial of dasatinib in CLL. In this study, Amrein et al. reported PR is 20%; moreover, notable nodal response is achieved more frequently than a reduction in peripheral blood leukocytosis.75 Dasatinib exhibits novel chemotherapeutic agents without a protective microenvironment.
From data of the phase II trial of dasatinib in relapsed and refractory CLL, we find dasatinib shows intense activity in high-risk del(11q) patients; at the same time, the toxicity of myelosuppression is frequently encountered. To date, dasatinib in combination with other drugs in CLL has not been performed in clinical trial except in vitro. Given studies providing support for efficacy in combination with other drugs in vitro, we believe combination therapy of dasatinib in CLL will become a fresh area in future studies.
Conclusion
Therapeutic approaches of targeting the microenvironment or microenvironment associated signaling in CLL is becoming as the most outstanding therapeutic strategy in B-cell malignancies. By targeting selected microenvironmental interactions mediated by the immune system in CLL, it could possibly disrupt the protective of malignant cells derived from cross-talk microenvironment, and as well create enhanced features with established therapeutics and overcome resistance mechanisms in high-risk and relapse CLL. A vast quantity of targeted microenvironmental interactions of clinical trials are ongoing and preliminary results are favorable. Clinical trial outcome of these targeted drugs are presented in Table 1. We should closely monitor durability time of responses, MRD, risk for disease progression, and long-term side effects. The development of these related targeted treatment, whether single agent or in combination with conventional therapeutics is supposed to improve the quality of life of CLL patients.
Table 1. Clinical efficacy of targeted environmental drugs in chronic lymphocytic leukemia.
| Agent | Current status | Target | Nodal decrease > 50% | Response rate by 2008 IW-CLL criteria | ||
|---|---|---|---|---|---|---|
| AMD310034 | Under clinic phase II | Inhibiting CXCL12/CXCR4 axis | - | 36% | ||
| GS-110150 | Under clinic phase III | Inhibiting PI3K signaling | 91% | 33% | ||
| Ibrutinib57 | Under clinic phase III | Inhibiting BTK signaling | 87% | 79% | ||
| Fostamatinib63 | Under clinic phase II | Inhibiting SYN signaling | 63% | 55% | ||
| Lenalidomide68 | Complete clinic phase II | Increasing bFGF and VEGF secretion; inhibiting PI3K signaling | 50% | 56% | ||
| Sorafenib71 | In malignant B cell lines | Blocking phosphorylation of ERK and MEK | - | - | ||
| Dasatinib75 | Complete clinic phase II | Inhibit BCR signaling | 44.4% | 20% |
IW-CLL, International Workshop on Chronic Lymphocytic Leukemia; -, not provided
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by National Natural Science Foundation of China (30971296, 81170488, 81370657), Natural Science Foundation of Jiangsu Province (BK2010584), Key Projects of Health Department of Jiangsu Province (K201108), Jiangsu Province’s Medical Elite Program (RC2011169), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institute (JX10231801), National Public Health Grand Research Foundation (201202017), the Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU, Jiangsu Province Higher Education Institute Foundation of Science and Technology Innovation Team Program, and the Project for State Key Clinical Department construction.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/26607
References
- 1.Doubek M, Mayer J, Obrtlíková P, Smolej L, Cmunt E, Schwarz J, Brejcha M, Kozmon P, Pospíšilová S, Brychtová Y, et al. Modern and conventional prognostic markers of chronic lymphocytic leukaemia in the everyday haematological practice. Eur J Haematol. 2011;87:130–7. doi: 10.1111/j.1600-0609.2011.01639.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K, Werner L, Sivachenko A, DeLuca DS, Zhang L, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365:2497–506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagatys EM, Zhang L. Clinical and laboratory prognostic indicators in chronic lymphocytic leukemia. Cancer Control. 2012;19:18–25. doi: 10.1177/107327481201900103. [DOI] [PubMed] [Google Scholar]
- 4.Herishanu Y, Pérez-Galán P, Liu D, Biancotto A, Pittaluga S, Vire B, Gibellini F, Njuguna N, Lee E, Stennett L, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–74. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010;115:1755–64. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, Garcia-Prieto C, Lu W, Burger JA, Croce CM, Plunkett W, et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol. 2012;14:276–86. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–75. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz BZ, Polliack A. Cancer microenvironment, extracellular matrix, and adhesion molecules: the bitter taste of sugars in chronic lymphocytic leukemia. Leuk Lymphoma. 2011;52:1619–20. doi: 10.3109/10428194.2011.589551. [DOI] [PubMed] [Google Scholar]
- 9.Bäckman E, Bergh AC, Lagerdahl I, Rydberg B, Sundström C, Tobin G, Rosenquist R, Linderholm M, Rosén A. Thioredoxin, produced by stromal cells retrieved from the lymph node microenvironment, rescues chronic lymphocytic leukemia cells from apoptosis in vitro. Haematologica. 2007;92:1495–504. doi: 10.3324/haematol.11448. [DOI] [PubMed] [Google Scholar]
- 10.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- 11.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–63. [PubMed] [Google Scholar]
- 12.Kunkel SL, Strieter RM, Lindley IJ, Westwick J. Chemokines: new ligands, receptors and activities. Immunol Today. 1995;16:559–61. doi: 10.1016/0167-5699(95)80076-X. [DOI] [PubMed] [Google Scholar]
- 13.Lukacs NW, Oliveira SH, Hogaboam CM. Chemokines and asthma: redundancy of function or a coordinated effort? J Clin Invest. 1999;104:995–9. doi: 10.1172/JCI8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–29. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan XJ, Dozmorov I, Li W, Yancopoulos S, Sison C, Centola M, Jain P, Allen SL, Kolitz JE, Rai KR, et al. Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood. 2011;118:5201–10. doi: 10.1182/blood-2011-03-342436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, Kipps TJ, Burger JA. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–30. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa S, Sato T, Abe M, Nagai S, Onai N, Yoneyama H, Zhang Y, Suzuki T, Hashimoto S, Shirai T, et al. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J Exp Med. 2001;193:1393–402. doi: 10.1084/jem.193.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Shazly AE, Doloriert HC, Bisig B, Lefebvre PP, Delvenne P, Jacobs N. Novel cooperation between CX3CL1 and CCL26 inducing NK cell chemotaxis via CX3CR1: a possible mechanism for NK cell infiltration of the allergic nasal tissue. Clin Exp Allergy. 2013;43:322–31. doi: 10.1111/cea.12022. [DOI] [PubMed] [Google Scholar]
- 19.Burger JA, Quiroga MP, Hartmann E, Bürkle A, Wierda WG, Keating MJ, Rosenwald A. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113:3050–8. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409:635–49. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 21.Moll NM, Ransohoff RM. CXCL12 and CXCR4 in bone marrow physiology. Expert Rev Hematol. 2010;3:315–22. doi: 10.1586/ehm.10.16. [DOI] [PubMed] [Google Scholar]
- 22.Möhle R, Failenschmid C, Bautz F, Kanz L. Overexpression of the chemokine receptor CXCR4 in B cell chronic lymphocytic leukemia is associated with increased functional response to stromal cell-derived factor-1 (SDF-1) Leukemia. 1999;13:1954–9. doi: 10.1038/sj.leu.2401602. [DOI] [PubMed] [Google Scholar]
- 23.Majid A, Lin TT, Best G, Fishlock K, Hewamana S, Pratt G, Yallop D, Buggins AG, Wagner S, Kennedy BJ, et al. CD49d is an independent prognostic marker that is associated with CXCR4 expression in CLL. Leuk Res. 2011;35:750–6. doi: 10.1016/j.leukres.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Patten PE, Buggins AG, Richards J, Wotherspoon A, Salisbury J, Mufti GJ, Hamblin TJ, Devereux S. CD38 expression in chronic lymphocytic leukemia is regulated by the tumor microenvironment. Blood. 2008;111:5173–81. doi: 10.1182/blood-2007-08-108605. [DOI] [PubMed] [Google Scholar]
- 25.Vlad A, Deglesne PA, Letestu R, Saint-Georges S, Chevallier N, Baran-Marszak F, Varin-Blank N, Ajchenbaum-Cymbalista F, Ledoux D. Down-regulation of CXCR4 and CD62L in chronic lymphocytic leukemia cells is triggered by B-cell receptor ligation and associated with progressive disease. Cancer Res. 2009;69:6387–95. doi: 10.1158/0008-5472.CAN-08-4750. [DOI] [PubMed] [Google Scholar]
- 26.Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, Cao F, Niekro W, Kempe T, Henning KA, et al. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res. 2013;19:357–66. doi: 10.1158/1078-0432.CCR-12-2333. [DOI] [PubMed] [Google Scholar]
- 27.Burger JA. Chemokines and chemokine receptors in chronic lymphocytic leukemia (CLL): from understanding the basics towards therapeutic targeting. Semin Cancer Biol. 2010;20:424–30. doi: 10.1016/j.semcancer.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Schols D, Struyf S, Van Damme J, Esté JA, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–8. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandera A, Verga L, Perseghin P, Incontri A, Pioltelli PE, Gori A. Use of CXCR4-antagonist for haematopoietic stem cell mobilization in HIV-infected patients with haematological malignancies. AIDS. 2013;27:1037–9. doi: 10.1097/QAD.0b013e32835ecbcd. [DOI] [PubMed] [Google Scholar]
- 30.Zhang WB, Navenot JM, Haribabu B, Tamamura H, Hiramatu K, Omagari A, Pei G, Manfredi JP, Fujii N, Broach JR, et al. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40-4C are weak partial agonists. J Biol Chem. 2002;277:24515–21. doi: 10.1074/jbc.M200889200. [DOI] [PubMed] [Google Scholar]
- 31.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donzella GA, Schols D, Lin SW, Esté JA, Nagashima KA, Maddon PJ, Allaway GP, Sakmar TP, Henson G, De Clercq E, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–7. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 33.Burger JA, Stewart DJ. CXCR4 chemokine receptor antagonists: perspectives in SCLC. Expert Opin Investig Drugs. 2009;18:481–90. doi: 10.1517/13543780902804249. [DOI] [PubMed] [Google Scholar]
- 34.Andritsos LA, Byrd JC, Hewes B, Kipps TJ, Johns D, Burger JA. Preliminary results from a phase I/II dose escalation study to determine the maximum tolerated from a phase I/II dose escalation study to determine the maximum tolerated chronic lymphocytic leukemia. Haematologica. 2010;95:Abstract 0772 . [Google Scholar]
- 35.Dal Bo M, Bomben R, Zucchetto A, Del Poeta G, Gaidano G, Deaglio S, Efremov DG, Gattei V. Microenvironmental interactions in chronic lymphocytic leukemia: hints for pathogenesis and identification of targets for rational therapy. Curr Pharm Des. 2012;18:3323–34. doi: 10.2174/138161212801227078. [DOI] [PubMed] [Google Scholar]
- 36.Kostareli E, Gounari M, Agathangelidis A, Stamatopoulos K. Immunoglobulin gene repertoire in chronic lymphocytic leukemia: insight into antigen selection and microenvironmental interactions. Mediterr J Hematol Infect Dis. 2012;4:e2012052. doi: 10.4084/mjhid.2012.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mraz M, Kipps TJ. MicroRNAs and B cell receptor signaling in chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54:1836–9. doi: 10.3109/10428194.2013.796055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–75. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Efremov DG, Gobessi S, Longo PG. Signaling pathways activated by antigen-receptor engagement in chronic lymphocytic leukemia B-cells. Autoimmun Rev. 2007;7:102–8. doi: 10.1016/j.autrev.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Kenkre VP, Kahl BS. The future of B-cell lymphoma therapy: the B-cell receptor and its downstream pathways. Curr Hematol Malig Rep. 2012;7:216–20. doi: 10.1007/s11899-012-0127-0. [DOI] [PubMed] [Google Scholar]
- 41.Hoellenriegel J, Coffey GP, Sinha U, Pandey A, Sivina M, Ferrajoli A, Ravandi F, Wierda WG, O’Brien S, Keating MJ, et al. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia. 2012;26:1576–83. doi: 10.1038/leu.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O’Brien S, Chiorazzi N, Burger JA. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, Giese N, O’Brien S, Yu A, Miller LL, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–12. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burger JA. Targeting the microenvironment in chronic lymphocytic leukemia is changing the therapeutic landscape. Curr Opin Oncol. 2012;24:643–9. doi: 10.1097/CCO.0b013e3283589950. [DOI] [PubMed] [Google Scholar]
- 45.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, Pals ST, Spaargaren M. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–4. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 46.Buchner M, Baer C, Prinz G, Dierks C, Burger M, Zenz T, Stilgenbauer S, Jumaa H, Veelken H, Zirlik K. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010;115:4497–506. doi: 10.1182/blood-2009-07-233692. [DOI] [PubMed] [Google Scholar]
- 47.Fruman DA, Rommel C. PI3Kδ inhibitors in cancer: rationale and serendipity merge in the clinic. Cancer Discov. 2011;1:562–72. doi: 10.1158/2159-8290.CD-11-0249. [DOI] [PubMed] [Google Scholar]
- 48.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 49.Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, Jones J, Andritsos L, Puri KD, Lannutti BJ, et al. Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM, Nina WJ et al. CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110d Demonstrates Clinical Activity and Pharmacodynamic Efects in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. 52nd American society of hematology,2010, 116 Abstract. [Google Scholar]
- 51.Coutre SE, Leonard JP, Furman RR, Barrientos J C, de Vos S, Flinn IW, et al. Combinations of the Selective Phosphatidylinositol 3-Kinase-Delta (PI3Kdelta) Inhibitor GS–1101 (CAL-101) with Rituximab and/or Bendamustine Are Tolerable and Highly Active in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL): Results From a Phase I Study. 54th American society of hematology, 2012, 191 Abstract. [Google Scholar]
- 52.Davids MS, Brown JR. Targeting the B cell receptor pathway in chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:2362–70. doi: 10.3109/10428194.2012.695781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107:13075–80. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kil LP, de Bruijn MJ, van Hulst JA, Langerak AW, Yuvaraj S, Hendriks RW. Bruton’s tyrosine kinase mediated signaling enhances leukemogenesis in a mouse model for chronic lymphocytic leukemia. Am J Blood Res. 2013;3:71–83. [PMC free article] [PubMed] [Google Scholar]
- 55.Davids MS, Burger JA. Cell trafficking in chronic lymphocytic leukemia. Open J Hematol. 2012;3(S1) doi: 10.13055/ojhmt_3_S1_03.120221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoellenriegel J, O'Brien S, Keating MJ. In vivo inhibition of BCR activation in high-risk CLL patients on therapy with bruton’s tyrosine kinase inhibitor ibrutinib: Correlative studies from an ongoing phase 2 clinical trial. 54th American society of hematology, 2012,186 Abstract. [Google Scholar]
- 57.Brown JR. Ibrutinib (PCI-32765), the first BTK (Bruton’s tyrosine kinase) inhibitor in clinical trials. Curr Hematol Malig Rep. 2013;8:1–6. doi: 10.1007/s11899-012-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jan A. Burger, Michael J. Keating, William G. Wierda, et al. The Btk Inhibitor Ibrutinib (PCI-32765) in Combination with Rituximab Is Well Tolerated and Displays Profound Activity in High-Risk Chronic Lymphocytic Leukemia (CLL) Patients, 54th American society of hematology, 2012, 187 Abstract [Google Scholar]
- 59.Semichon M, Merle-Béral H, Lang V, Bismuth G. Normal Syk protein level but abnormal tyrosine phosphorylation in B-CLL cells. Leukemia. 1997;11:1921–8. doi: 10.1038/sj.leu.2400832. [DOI] [PubMed] [Google Scholar]
- 60.Gobessi S, Laurenti L, Longo PG, Carsetti L, Berno V, Sica S, Leone G, Efremov DG. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23:686–97. doi: 10.1038/leu.2008.346. [DOI] [PubMed] [Google Scholar]
- 61.Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, Qu K, Herlaar E, Lau A, Young C, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 62.Herman SE, Barr PM, McAuley EM, Delong Liu, Friedberg JW, Adrian Wiestner. Fostamatinib Inhibits BCR Signaling, and Reduces Tumor Cell Activation and Proliferation in Patients with Relapsed Refractory Chronic Lymphocytic Leukemia. 54th American society of hematology, 2012, 2882 Abstract. [Google Scholar]
- 63.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–85. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crane E, List A. Lenalidomide: an immunomodulatory drug. Future Oncol. 2005;1:575–83. doi: 10.2217/14796694.1.5.575. [DOI] [PubMed] [Google Scholar]
- 65.Menzel T, Rahman Z, Calleja E, White K, Wilson EL, Wieder R, Gabrilove J. Elevated intracellular level of basic fibroblast growth factor correlates with stage of chronic lymphocytic leukemia and is associated with resistance to fludarabine. Blood. 1996;87:1056–63. [PubMed] [Google Scholar]
- 66.Chen H, Treweeke AT, West DC, Till KJ, Cawley JC, Zuzel M, Toh CH. In vitro and in vivo production of vascular endothelial growth factor by chronic lymphocytic leukemia cells. Blood. 2000;96:3181–7. [PubMed] [Google Scholar]
- 67.Herman SE, Lapalombella R, Gordon AL, Ramanunni A, Blum KA, Jones J, Zhang X, Lannutti BJ, Puri KD, Muthusamy N, et al. The role of phosphatidylinositol 3-kinase-δ in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood. 2011;117:4323–7. doi: 10.1182/blood-2010-11-315705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N, Kukreti V, Wei E, Leung-Hagesteijn C, Li ZH, et al. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol. 2011;29:1175–81. doi: 10.1200/JCO.2010.29.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Badoux XC, Keating MJ, Wen S, Wierda WG, O’Brien SM, Faderl S, Sargent R, Burger JA, Ferrajoli A. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2013;31:584–91. doi: 10.1200/JCO.2012.42.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rini BI. Sorafenib. Expert Opin Pharmacother. 2006;7:453–61. doi: 10.1517/14656566.7.4.453. [DOI] [PubMed] [Google Scholar]
- 71.Messmer D, Fecteau JF, O’Hayre M, Bharati IS, Handel TM, Kipps TJ. Chronic lymphocytic leukemia cells receive RAF-dependent survival signals in response to CXCL12 that are sensitive to inhibition by sorafenib. Blood. 2011;117:882–9. doi: 10.1182/blood-2010-04-282400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindauer M, Hochhaus A. Dasatinib. Recent Results Cancer Res. 2010;184:83–102. doi: 10.1007/978-3-642-01222-8_7. [DOI] [PubMed] [Google Scholar]
- 73.McCaig AM, Cosimo E, Leach MT, Michie AM. Dasatinib inhibits B cell receptor signalling in chronic lymphocytic leukaemia but novel combination approaches are required to overcome additional pro-survival microenvironmental signals. Br J Haematol. 2011;153:199–211. doi: 10.1111/j.1365-2141.2010.08507.x. [DOI] [PubMed] [Google Scholar]
- 74.McCaig AM, Cosimo E, Leach MT, Michie AM. Dasatinib inhibits CXCR4 signaling in chronic lymphocytic leukaemia cells and impairs migration towards CXCL12. PLoS One. 2012;7:e48929. doi: 10.1371/journal.pone.0048929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amrein PC, Attar EC, Takvorian T, Hochberg EP, Ballen KK, Leahy KM, Fisher DC, Lacasce AS, Jacobsen ED, Armand P, et al. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res. 2011;17:2977–86. doi: 10.1158/1078-0432.CCR-10-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]