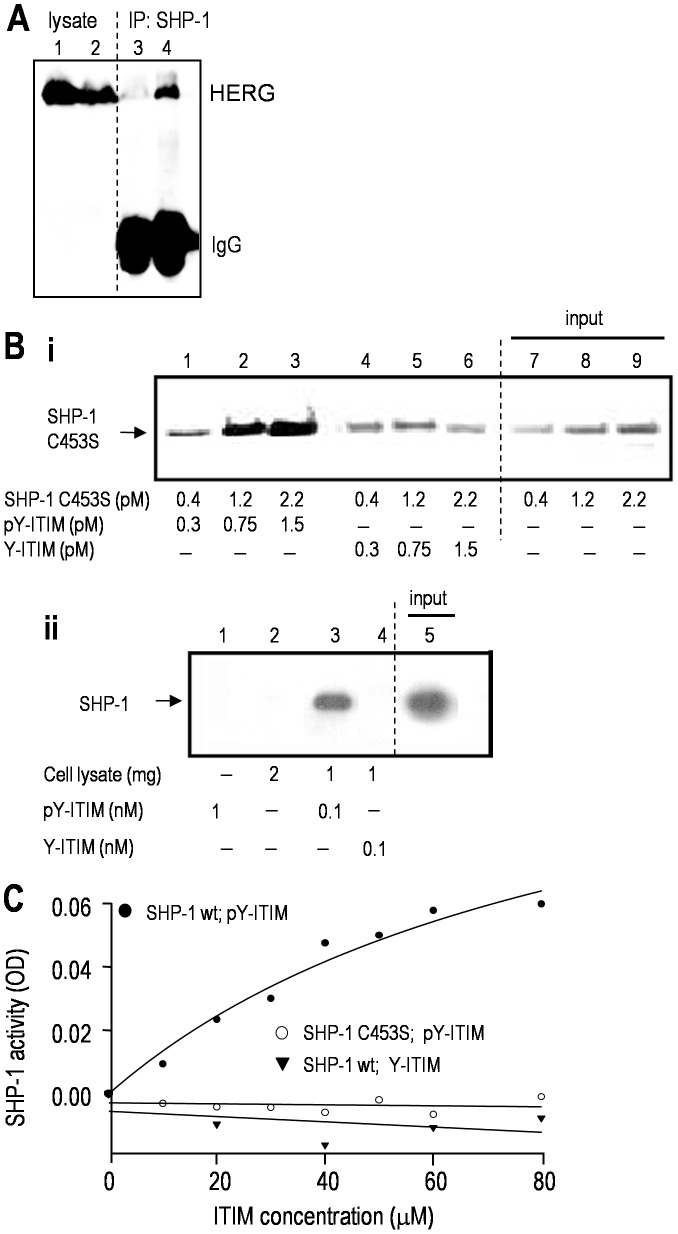
Figure 6. The ITIM of hERG/Kv11.1 binds to, and trans-activates SHP-1 tyrosine phosphatase.
A. The ITIM motif of hERG is required for its interaction with SHP-1. HEK293 cells expressed either wt hERG (lanes 1&4) or ΔITIM hERG (lanes 2&3). Immunoprecipitation: anti-SHP-1 antibody; Western blots: anti-hERG antibody. The lysate lanes show that channel protein expression was not affected by ITIM deletion. B. Tyrosine phosphorylation of the hERG ITIM is required for its direct binding to SHP-1. i. Purified recombinant SHP-1 C453S protein was incubated with increasing concentrations of the phosphorylated (pY-ITIM; lanes 1–3) or non-phosphorylated (Y-ITIM; lanes 4–6) ITIM-hERG peptide (see Methods). The peptides, which were biotinylated, were pulled down with avidin-coated beads. The Western blot was probed with anti-SHP-1 antibody, and lanes 7–10 show the input SHP-1 C453S protein. ii. Pull-down of endogenous SHP-1. Lysates from MLS-9 cells, which express SHP-1 (lane 5; 80 µg of input lysate), were incubated with each biotinylated ITIM-hERG peptide, which was pulled down with avidin-coated beads and probed for SHP-1, as above. C. The SHP-1 enzyme is activated by the ITIM of hERG. Phosphorylated or non-phosphorylated ITIM-hERG peptides were incubated for 3 h with 0.2 µg of wt SHP-1 in a buffer containing 10 mM pNPP (p-nitrophenyl phosphate) as the substrate (see Methods). Similarly, the tyrosine phosphorylated ITIM-hERG peptide was incubated with SHP-1 C453S.