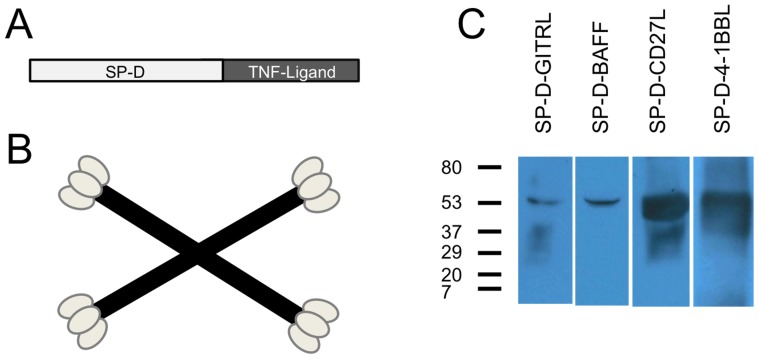
Figure 1. Construction of SP-D-TNFSFL Adenoviral vectors.
A) Illustration of SP-D-TNFSFL cloning strategy. SP-D-TNFSFL genes were cloned by fusing the SP-D collagen-like domain to the extracellular domain of each ligand. Amino acids 1-256 of surfactant protein D were fused to the extracellular domain of each TNFSFL gene, including 4-1BBL, GITRL, BAFF, and CD27L. Details are provided in Materials and Methods B) Diagram of SP-D-TNFL multi-trimer structure. SP-D spontaneously forms four trimeric “arms” that are linked through disulfide bonding at the N-terminus to form 4-trimer structures [48], [49]. Shown here is a cartoon of the 4-trimer structure spontaneously formed by the collagen-like domain of SP-D followed by disulfide bonding of the SP-D N-terminus. C) Western blot of 293 cells infected with Ad5 constructs expressing SP-D-TNFL. 48 hours following viral vector transduction, supernatants were collected and run on a 4–15% Tris-Glycine SDS-PAGE gel in the presence of DTT before being probed with respective antibodies (see materials and methods). All viruses produced a 50–55 kDa recombinant protein expressing the expected TNF superfamily ligand.